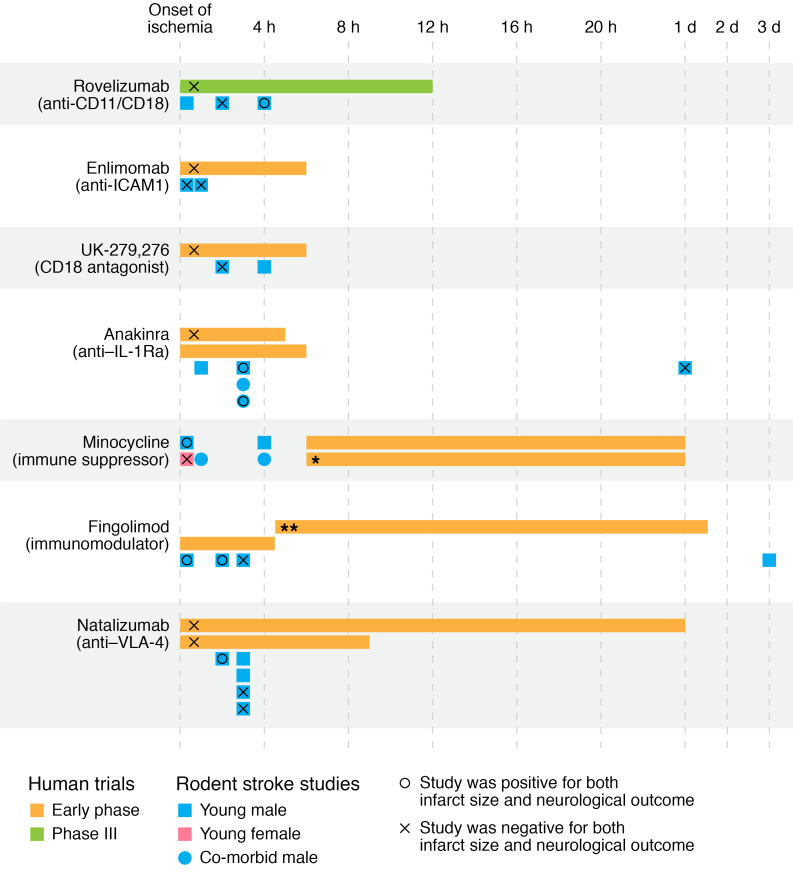
Figure 3. Timing and success of selected immunomodulatory therapies for stroke.
Human studies were selected as those that were later stage and utilized immunomodulatory drugs or antibodies. For each agent, the human studies are listed in chronological order, with the length of the bars indicating the treatment period from the time the participant was last seen normal. Below the human trials are animal studies with that agent where the first dose was delivered after stroke, and either infarct size or neurological outcome was tested. If both were positive, the animal studies are marked with a circle, and if both were negative, they are marked with an x. In cases where only neurological outcome or only stroke size was tested, or where one was positive and the other negative, the study is not marked with a symbol. Comorbidities in animal studies were aging, diabetes, hypertension, and hypercholesterolemia. Additional details and references are in Supplemental Table 1. *This study had a positive effect in males but not females. **Drug dose timing listed only as mean ± SD, which is graphed here.