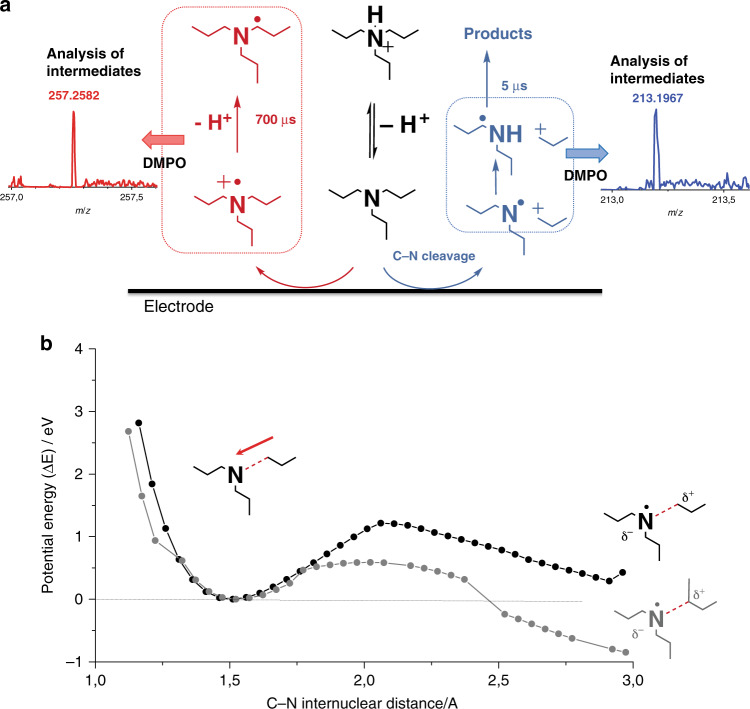
Fig. 3. Proposed mechanism for tri-n-propylamine (TPrA) and dipropylamine (DPrA) radical generation.
a Schematic representation of the proposed parallel pathways for the tri-n-propylamine (TPrA) oxidation at the electrode where TPrA radical cation and radical are generated (red pathway) and where dipropylamine radical (DPrA) is generated (blue pathway). The scheme reaction is supported by spin-trapping experiments with 5,5-dimethyl-pyrroline N-oxide (DMPO), which stabilized the radicals and allowed identification by mass spectrometry analysis (MS) and electron paramagnetic resonance (EPR). The inset in a shows the MS analysis for the possible adducts DMPO-TPrA and DMPO-DPrA (see “Supplementary Methods 3” for the detailed mechanism and Supplementary Fig. 12 and Supplementary Tables 2 and 3). b Potential energy curve along C–N (dashed red bond in the depicted molecular structures) stretching of TPrA (black curve and dots) and DPIBA (gray curve and dots) computed with UM062X/6-31G* in H2O (described with IEFPCM method at each point of the curve, the energy of the equilibrium structure has been subtracted). The calculations are run in the presence of an external electric field (~108 V cm−1) whose orientation is represented by the red arrow. Under the effect of the external electric field the curves acquire a typical reaction profile. The mechanism shows a prevailing ionic character with the formation of a carbocation propyl fragment (represented by the δ+ charge on the molecular structures).