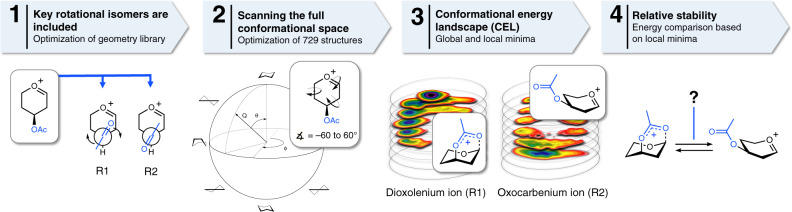
Fig. 4. Overview of the workflow to map the relative stability of glycosyl dioxolenium- and oxocarbenium ions.
(1) Two rotamers are used to probe long-range participation: R1 makes it geometrically feasible to form dioxolenium ions, where R2 generates the free oxocarbenium ion. (2) The complete conformational space of the 6-membered rings was scanned by computing 729 prefixed structures per rotamer; A few canonical conformations (chair, half-chair, envelope, and boat) are depicted. (3) The associated energies were graphed on slices dividing the Cremer–Pople sphere; the CEL map of the R1 rotamer and CEL map of the R2 rotamer. (4) Based on the CEL maps of R1 and R2 the relative stability of both intermediates can be evaluated.