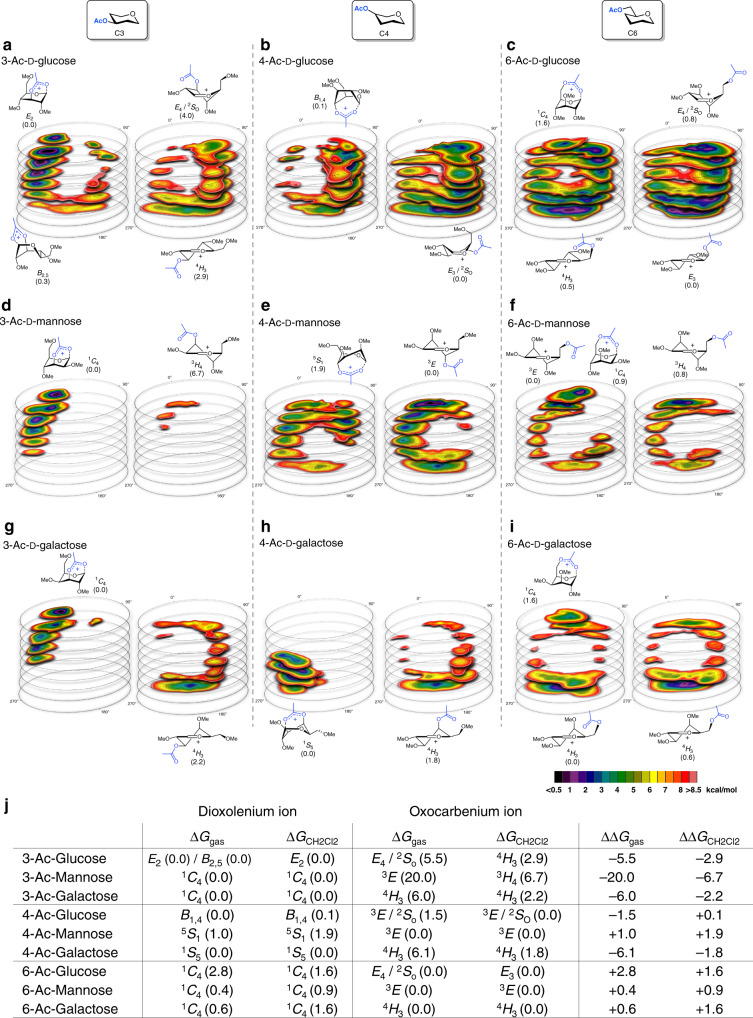
Fig. 5. CEL maps of selected glycosyl cations in which the local minima identified are shown with their respective energy42.
Two acetyl ester rotamers (R1 and R2) were considered for all computed glycosyl cations generating two sperate CEL maps. All energies are as computed at PCM(CH2Cl2)-B3LYP/6-311G(d,p) at 213.15 K and expressed as solution-phase Gibbs free energy. CEL maps for C3-acetyl pyranosyl ions (a–c), C4-acetyl pyranosyl ions (d–f), C6-acetyl pyranosyl ions (g–i). j Table summarizing the relative energy of the dioxolenium and oxocarbenium ion conformers in the gas- and solution-phase.