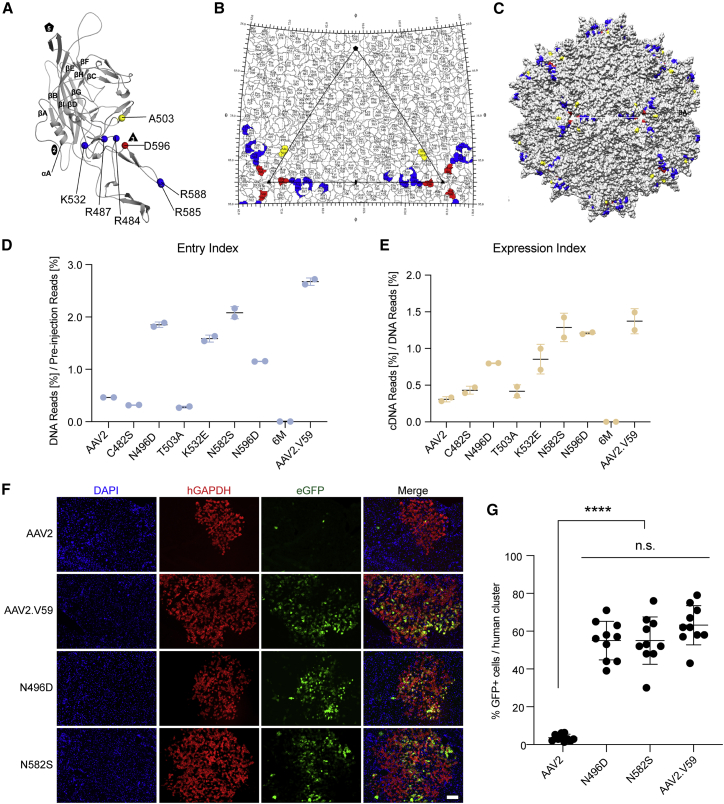
Figure 3.
Fnctional analysis of HSPG-detargeted AAV2 variants in the hFRG mice.
(A–C) Structure and location of AAV2.V5 residues. (A) Model of VP3 monomer colored gray. The residues involved in heparin binding are R484, R487, K532, R585, and R588, and they are colored blue. Two residues important for reduced heparin binding and improved hepatotropic transduction are D596 (red) and A503 (yellow). (B and C) Surface map (B) and stereographic roadmap projection (C) of the 3D model viewed down the icosahedral 2-fold axes. The icosahedral 2-fold, 3-fold, and 5-fold axes are depicted as an oval, triangle, and pentagon, respectively. (D and E) Relative in vivo performance of AAV2 variants in the hFRG model, represented as (D) entry (499.3 vg/diploid cell) and expression (E) indexes. (F) Representative immunohistochemical analysis of the liver of an hFRG mouse transduced with AAV2, AAV2.V59, AAV2-N5496D, and AAV2-N582S variants. Statistical significance was calculated using the two-tailed Mann-Whitney test, comparing the performance of each novel variant with AAV2. Red, human GAPDH; green, vector-encoded GFP; blue, DAPI (nuclei). Scale bar, 100 μm. (G) Quantification of the percentage of transduced human hepatocytes per human cluster. Data are shown as mean ± SD (n = 10 human clusters/mouse, n = 1 mouse/vector). Statistical significance was calculated using the two-tailed Mann-Whitney test. ∗∗∗∗p < 0.0001. n.s., not significant (p > 0.05).