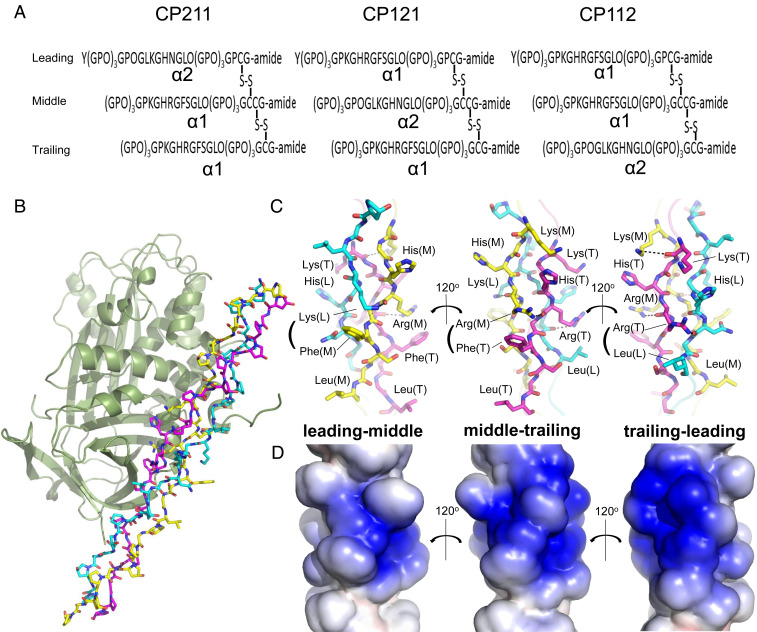
Fig. 1.
Structural determination of PEDF in complex with synthetic heterotrimeric type I collagen peptide. (A) Designed disulfide-crosslinked heterotrimeric collagen peptides with three combinations of chain arrangements, in which the α2 chain segment derived from a sequence alignment with the previously identified PEDF-binding sequence of type I collagen α1 chain is positioned at the leading, middle, or trailing position (i.e., α2α1α1, α1α2α1, or α1α1α2), designated CP211, CP121, and CP112, respectively. (B) The PEDF-CP211 complex shown as a cartoon for PEDF and a stick model for CP211. The leading (L), middle (M), and trailing (T) chains in the triple helix are colored light blue, yellow, and magenta, respectively. (C) Stick model of the guest region of CP211 shown in three different orientations, each rotated by 120° about the vertical axis; highlighted leading-middle chains, middle-trailing chains, and trailing-leading chains are shown (left to right). Basic (Lys, His, and Arg) and hydrophobic (Phe and Leu) residues are labeled. Hydrogen-bonding interactions are shown as dotted lines. (D) Electrostatic potential surface representation of the guest region of CP211. The three different orientations, each rotated by 120° about the vertical axis, are shown as in Fig. 1C.