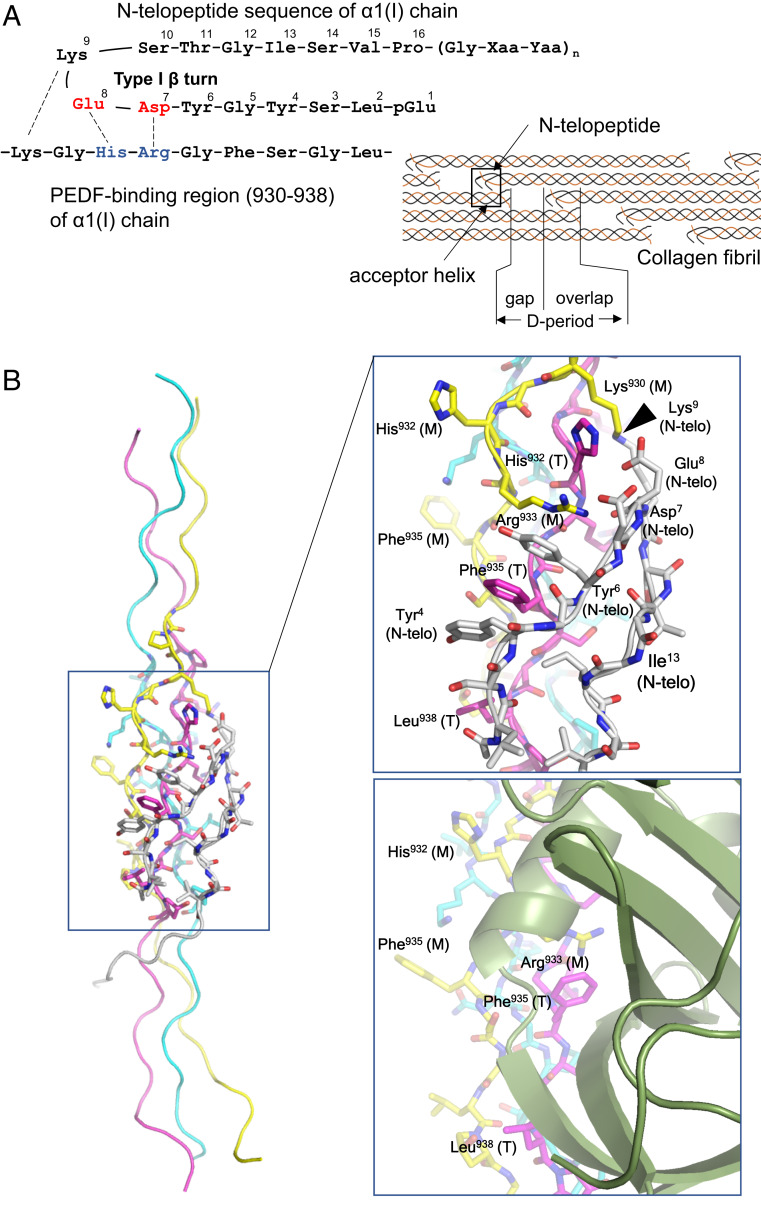
Fig. 3.
Docking model of CP211 and the N-telopeptide segment of type I collagen. (A, Left) Schematic of interactions between the N-telopeptide and the PEDF-binding region of the α1 chain of type I collagen. (A, Right) Schematic of the axial arrangement of type I collagen molecules in the D-periodic fibril. The N-telopeptide segment and the PEDF-binding region (also termed the “acceptor helix”) are indicated by arrows. (B, Left) Overall structure of the CP211–N-telopeptide docking model. (B, Right, Top) Closeup view of the CP211–N-telopeptide interaction surface. The leading (L), middle (M), and trailing (T) chains in the triple helix are colored light blue, yellow, and magenta, respectively. The N-telopeptide segment is in white. Some amino acid residues of the guest region of CP211, located at the interface between CP211 and N-telopeptide, are labeled and numbered according to their positions in the type I collagen sequence. In addition to electrostatic interactions, the intercalation of Phe935 with two tyrosyl residues of the N-telopeptide, which is stabilized by aromatic ring stacking, is a hallmark of the interaction. The site of intermolecular crosslinking is indicated by an arrowhead. (Right, Bottom) Closeup view of the PEDF-CP211 interaction surface shown for comparison.