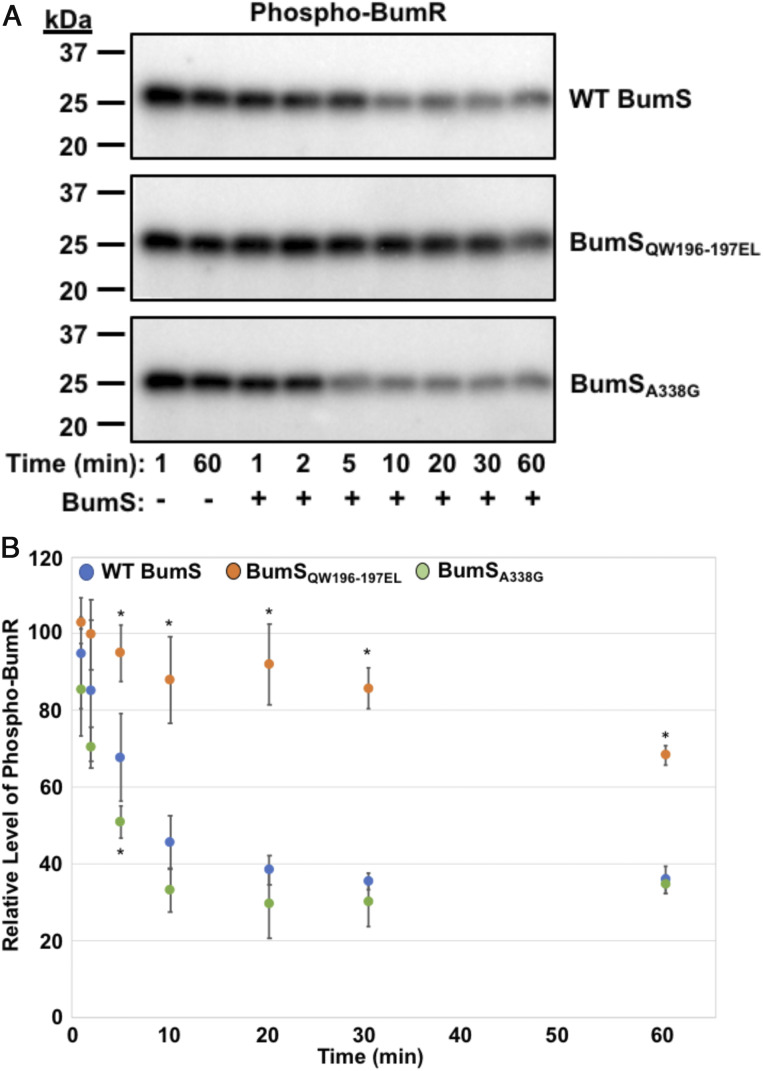
Fig. 7.
BumS phosphatase activity is dependent on specific H box residues. (A and B) In vitro phosphatase activities WT BumS, BumSQW196-7EL (an H box mutant), and BumSA338G (a D box mutant) over time. After autophosphorylation of BumR by Ac[32P], BumS proteins were added at 1:2 BumS:BumR molar ratios for up to 60 min. Reactions were stopped at various times indicated. (A) Representative phosphatase assay of WT BumS or BumS mutants for phospho-BumR. (B) Quantification of three independent phosphatase assays for WT BumS, BumSQW196-7EL, and BumSA338G. The level of phospho-BumR in each assay was normalized to phospho-BumR at 1 min of mock sample, which was set to 100%. No appreciable decrease in phospho-BumR in the absence of BumS was observed for the duration of the experiment (up to 60 min) as observed in A. Error bars indicate SDs of the relative level of phospho-BumR at indicated time after addition of BumS from three independent experiments. Statistical significance to compare WT to mutant proteins was calculated in GraphPad Prism by ANOVA with Dunnett’s test (*P < 0.05).