Figure 3. Lipid metabolism in RA and SLE T cells.
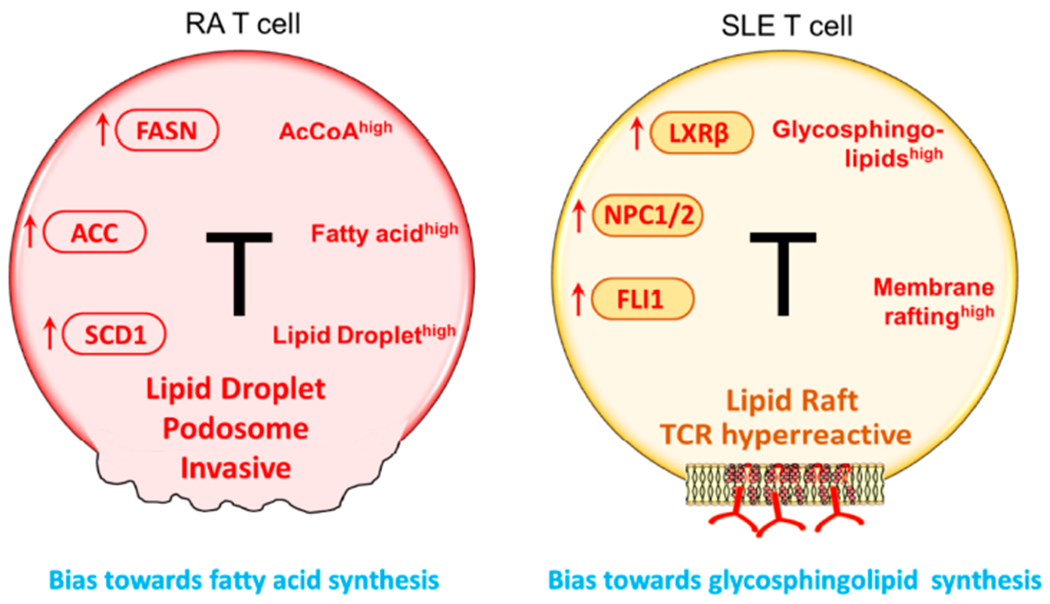
Despite their ATPlow status, RA T cells activate the lipogenesis machinery. Enzymes supporting de novo fatty acid synthesis, including FASN, ACC, and SCD1 are all upregulated. Abundancy in intracellular AcCoA and NADPH facilitate the shift towards lipid generation. As a result, lipid droplets accumulate in the cytoplasm where they serve as a reservoir of biosynthetic activity. Equipped with surplus lipids, RA T cells tend to form membrane extensions; podosome-like structure that enable T cells to invade into matrix and promote tissue inflammation. In SLE T cells, glycosphingolipids (GSLs) synthesis is enhanced. Oversupply in GSLs changes the plasma membrane composition and structure, promoting lipid rafts formation. One outcome of membrane rafting is the enhancement of TCR signaling, enabled by easing the recruitment of lipid raft-associated signaling mediators, such as the proteins of the TCR complex and downstream signaling molecules. FASN, fatty acid synthase; ACC, acetyl-CoA carboxylase; SCD1, stearoyl-CoA desaturase; TCR, T cell receptor.
