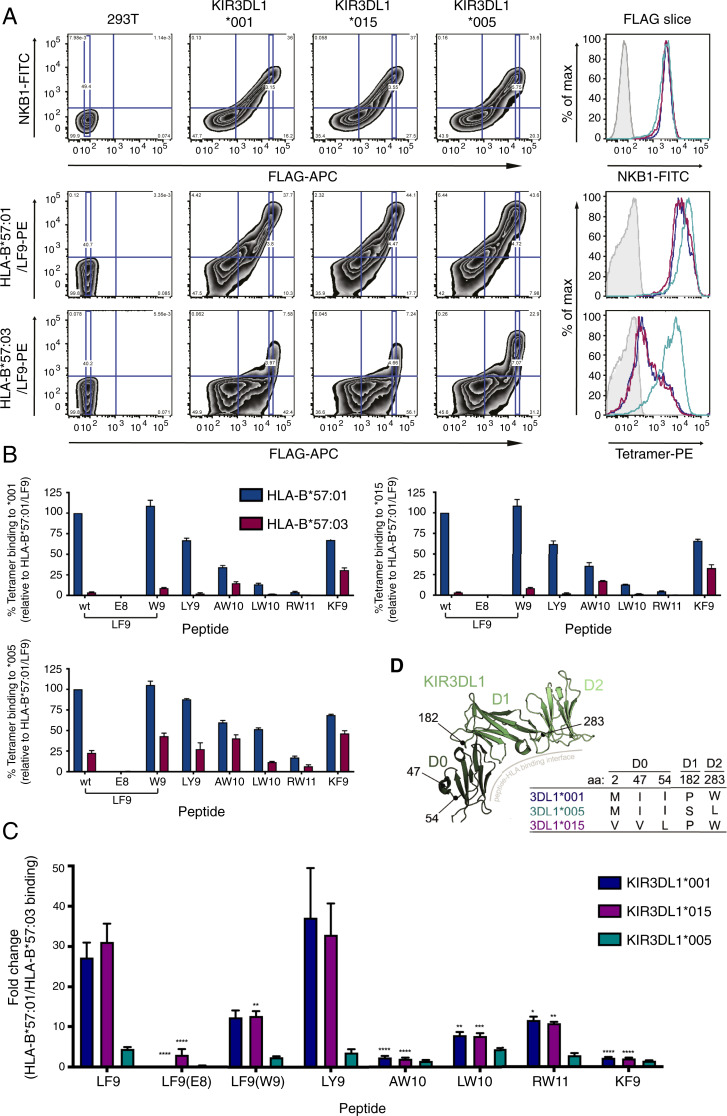
Fig. 2.
KIR3DL1*005 displays broader binding to HLA-B*57:03 regardless of peptide. 293T cells were transfected with FLAG-tagged KIR3DL1*001, *015, or *005 constructs, and the binding of HLA-B*57:01 and HLA-B*57:03 tetramers loaded with the peptides LF9(wild-type; wt), LF9(E8), LF9(W9), LY9, AW10, LW10, RW11, and KF9 was assessed 48 h later. Surface expression of KIR3DL1 at equal FLAG intensities (FLAG slice) was confirmed by staining with NKB1-FITC. Transfectants were stained with tetramers at equivalent MFI values (based on LILRB1 binding) and for FLAG. Tetramer binding values were obtained at a given FLAG-APC MFI (FLAG slice). (A) Representative flow cytometry plots for NKB1-FITC, HLA-B*57:01/LF9-PE tetramer, and HLA-B*57:03/LF9-PE staining versus FLAG-APC staining, with the corresponding MFI of the FLAG-slice shown in histogram form (unstained, filled; SA-PE only, light gray; *001, blue; *015, purple; and *005 teal). (B) Staining of HLA-B*57:01 and HLA-B*57:03 tetramers with given peptides to KIR3DL1*001, *015, or *005–expressing 293T cells at the selected FLAG MFI. Tetramer staining is normalized to HLA-B*57:01 with wt LF9 as a known positive control from three independent experiments (error bars represent SEM). (C) The fold change between the MFI of HLA-B*57:01 and HLA-B*57:03 tetramer binding at the given FLAG intensity. Error bars represent the SEM, and asterisks indicate a statistical difference from the wt LF9 peptide for each respective KIR3DL1 allotype as calculated with a two-way ANOVA with Tukey’s multiple comparison test (*P < 0.1, **P < 0.01, ***P < 0.001, ****P < 0.0001). (D) Overview of KIR3DL1 polymorphisms in alleles tested (*001, *005, and *015) for HLA-B57 tetramer binding. Amino acid position 283 is proximal to the peptide-HLA binding interface in contrast to positions 47, 54, and 182. Amino acid usage in each position and their domain positions are indicated by the Inset table. Generated using PDB accession code 3VH8 (12).