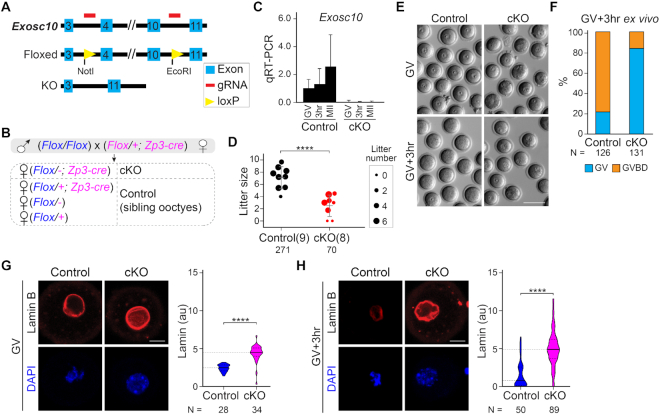
Figure 1.
Oocyte-specific knockout of Exosc10 causes female subfertility by impairing GVBD during oocyte maturation. (A) Schematic of strategy to generate an Exosc10 floxed allele using CRISPR/Cas9. Two loxP sites were inserted to bracket exons 4–10. (B) Mating strategy to obtain oocyte-specific conditional knockouts of Exosc10 (cKO). Siblings with other genotypes were used as controls. The paternal allele is labeled in blue and the maternal allele is labeled in magenta in the offspring. Note that the floxed maternal Exosc10 allele will become a deletion allele (−) during oocyte growth. (C) qRT-PCR of Exosc10 in single oocytes obtained from controls and cKO mice. Error bars: standard deviation of three technique replicates of each sample. (D) Dot plot of individual litter sizes over 6 months of harem breeding of controls and cKO females with wild-type males. The sizes of the dots are normalized by the average litter number per female. The number in parenthesis is the number of females having the indicated genotypes. The number of pups born is indicated below each group. The horizontal lines represent the mean and standard deviation. (E) Bright-field images of cKO and control oocytes cultured ex vivo for 0 (GV) or 3 h (GV3h). (F) Percentage of GVBD oocytes in E. Numbers of oocytes are indicated below each group. (G and H) Confocal fluorescence and DAPI images of oocytes after lamin B immunostaining at GV (G) and GV3h (H) stages. Lamin B and DAPI are maximum intensity projections. Quantification of lamin B fluorescence is on the right. The horizontal lines inside the violins represent the median and the quartiles. The number of oocytes from at least three females are indicated below each group. **** P < 0.0001 in D, G, H, two-tailed Student's t-test. Scale bars: 100 μm in E; 20 μm in G and H.