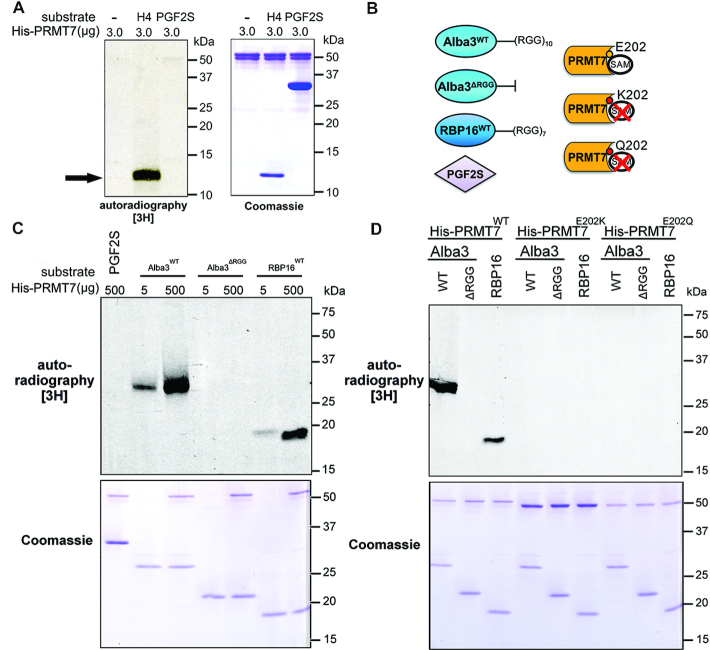
Figure 4.
In vitro methylation of Alba3 and RBP16 by PRMT7. (A) Recombinant His-PRMT7 was tested for methyltransferase activity in vitro in the presence of radioactive methyl donor S-adenosyl methionine, human histone H4 as (1 μg) a canonical RGG-containing PRMT substrate (black arrow) and a Leishmania braziliensis protein rich in arginines but devoid of RGG motifs as a negative control (LbrPGF2S). (B) Purified His-tagged PRMT7 putative substrates Alba3WT, RBP16WT and RGG-deficient Alba3ΔRGG were tested, as well as LbrPGF2SWT. PRMT7 E202 residue in the double E loop motif was mutated to K or Q to generate catalytically-inactive mutants. (C) RNA-binding proteins Alba3WT and RBP16 are arginine methylated by PRMT7 in vitro, while Alba3ΔRGG and LbrPGF2SWT are not. (D) In vitro methylation of target RBPs by PRMT7 is disrupted by mutating residue E202 (PRMT7E202K and PRMT7E202Q). Coomassies demonstrate relative loading.