Figure 9.
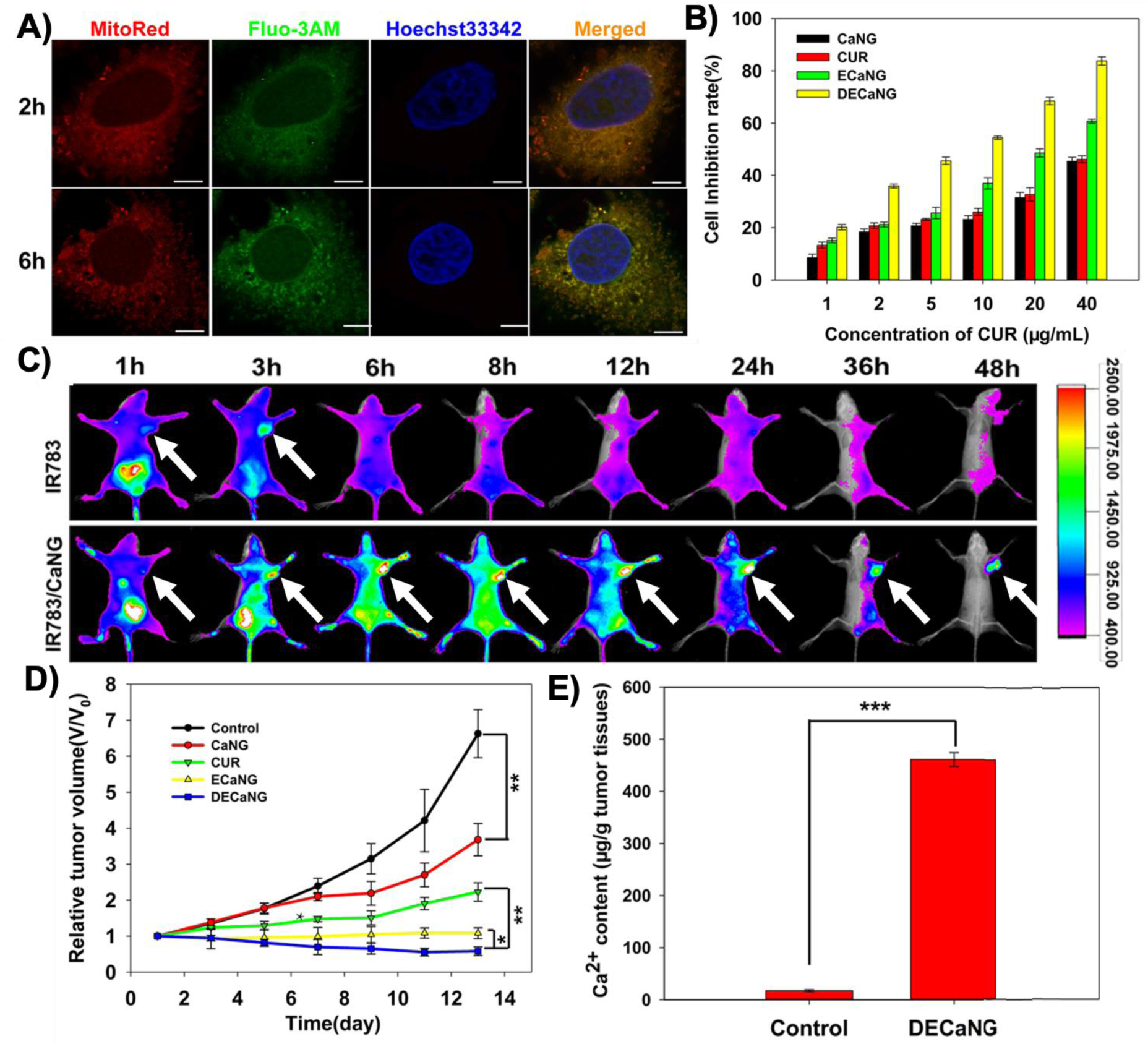
(A) Confocal fluorescent images of the MCF-7 breast cancer cells showing distribution of Ca2+ (stained with Fluo-3AM) released from degraded CaP nanoparticles (so called calcium nanogenerators or CaNG) in mitochondria (stained with MitoRed) at different incubation times (2 and 6 h). Hoechst33342 was used for staining the nucleus. (Scale bars: 7.5 μm) (B) Degradation of the CaP nanoparticles disrupted the mitochondrial Ca2+ homeostasis and resulted in enhanced apoptosis (growth inhibition) of the cancer cells. (C) In vivo fluorescent imaging at different post-injection times indicated these nanoparticles could accumulate in subcutaneous tumors (shown by white arrows), when they were tagged with IR783 fluorescent molecules, compared with free IR783 molecules. (D) Mitochondrial disruption of the Ca2+ homeostasis helped to inhibit tumor growth and enhanced the therapeutic effects, especially when combined with photothermal therapy. (E) Significantly higher amount of Ca2+ in tumor tissues obtained from the mice treated with DECaNG compared with control mice only injected with saline. CaNG: the synthetic CaP nanoparticles; CUR: curcumin, an agent to enhance release of Ca2+ from endosomes to cytoplasm; ECaNG: enhanced Ca2+ nanogenerators or CUR-loaded CaNGs;DECaNG: dual enhanced Ca2+ nanogenerator, CaNG loaded with both CUR and a fluorescent molecule for simultaneous Ca2+ release and photothermal therapy effects. (Adopted with permission from ref. [58]. Copyright 2018 American Chemical Society)
