Fig. 4. The N-terminal domain of NADK attenuates its catalytic activity.
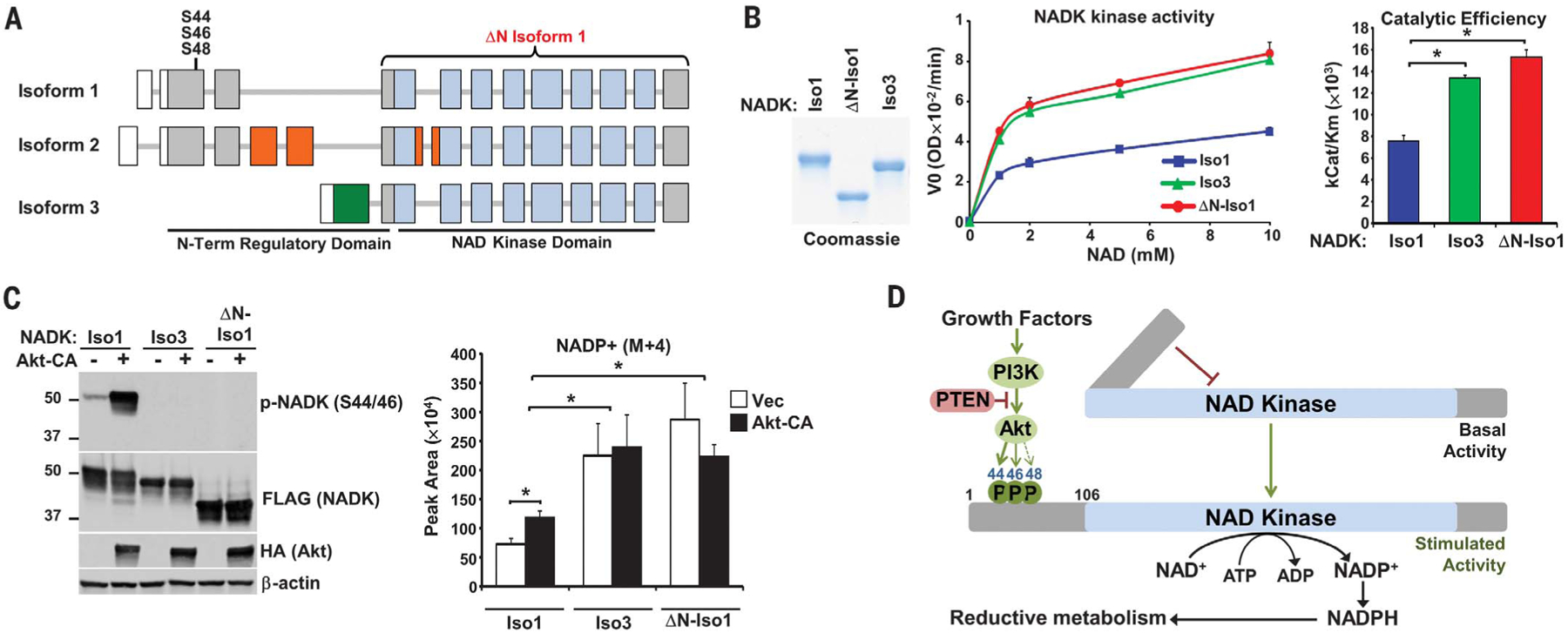
(A) Schematic of NADK isoform exons, with untranslated regions (white), kinase domain (blue), and regions unique to isoforms 2 (orange) and 3 (green) depicted. (B) NADK isoforms immunopurified from serum-deprived HEK293E cells were subjected to Coomassie staining and an NADK activity assay. Data are presented as the mean ± SD of biological duplicates and are representative of three independent experiments. *P < 0.05. (C) HEK293E cells with stable shRNA-mediated knockdown of NADK were co-transfected with NADK isoforms and either empty vector (−) or Akt-CA, serum-deprived overnight, and labeled for 30 min with 13C3-15N-nicotinamide. Normalized peak areas are presented as the mean ± SD of biological quadruplicates and are representative of two independent experiments. *P < 0.05. (D) Model of NADK activation by Akt signaling.
