Figure 7.
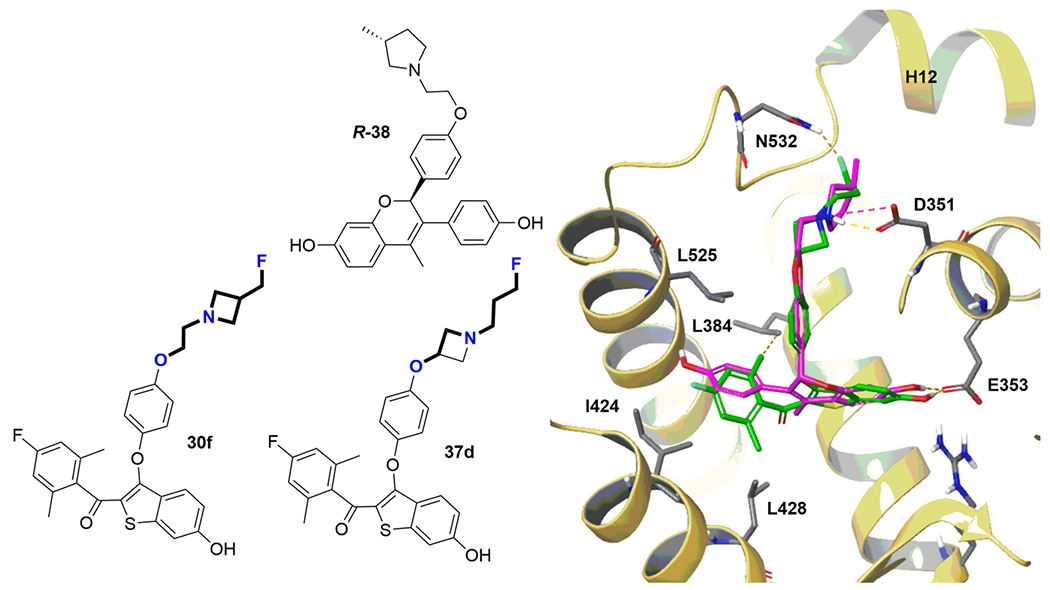
B-SERD docking to ER. B-SERDs 30f and 37d have a common basic side-arm motif: 3-fluoro-N-(2-oxyethyl)propan-1-amine. The structure design envisaged the occupation of the hydrophobic pockets formed by leucines 384, 428, 525, and isoleucine 424 by the substituted benzoyl ring, allowing a salt bridge interaction between the amine side arm amine and Asp-351 in the ERα ligand binding site. B-SERD 37d (green) was docked to the ligand binding domain of ERα obtained from the co-crystal structure with the SERD R-38 (magenta) (pdb 5UFX) confirming the proposed binding site interactions leading to destabilization of H12.
