Scheme 1. Synthetic Routes for Precursor Synthonsa.
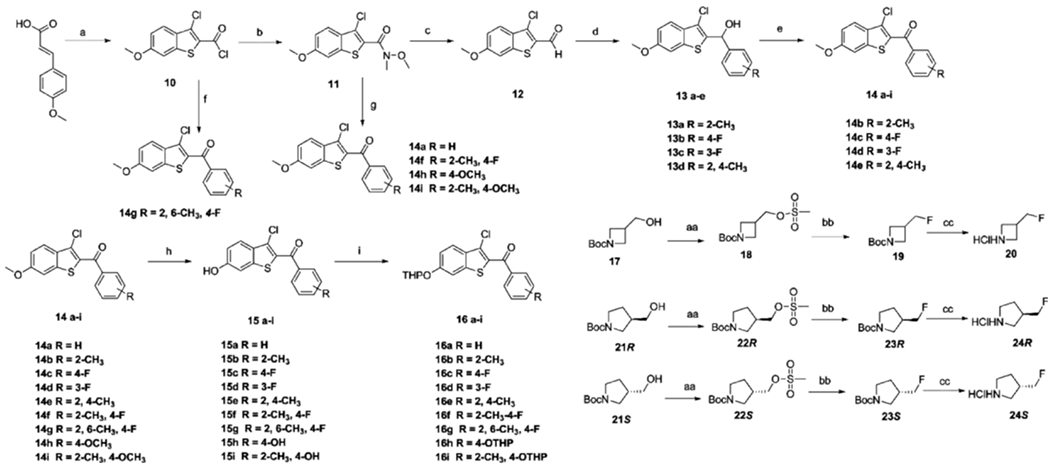
aReagents and conditions: (a) SOCI2, pyridine, chlorobenzene, reflux, 50%. (b) N-Methoxymethylamine, Et3N, DCM, rt, 90%. (c) DIBAL-H, THF, −40 °C, 60%. (d) Grignard reagent, THF, 0 °C to rt, 75–85%. (e) PCC, DCM, rt, 55–65%. (f) (4-Fluoro-2,6-dimethylphenyl)magnesium bromide, CuCN-2LiCl, THF, 0 °C to rt, 90%. (g) Grignard reagent, THF, 0 °C to rt, 70–80%. (h) BBr3, DCM, −78 °C to rt, 40–60%. (i) 3,4-Dihydro-2H-pyran, PPTS, DCM, rt, 70–80%. (aa) Methanesulfonyl chloride, Et3N, DCM, 0 °C to rt, 95%. (bb) TBAF, 80 °C, 70%. (cc) 6 M HCI MeOH, rt, 60%.
