Scheme 2. Synthetic Routes for Candidate Piperidine, Azetidine, and Pyrrolidine B-SERDsa.
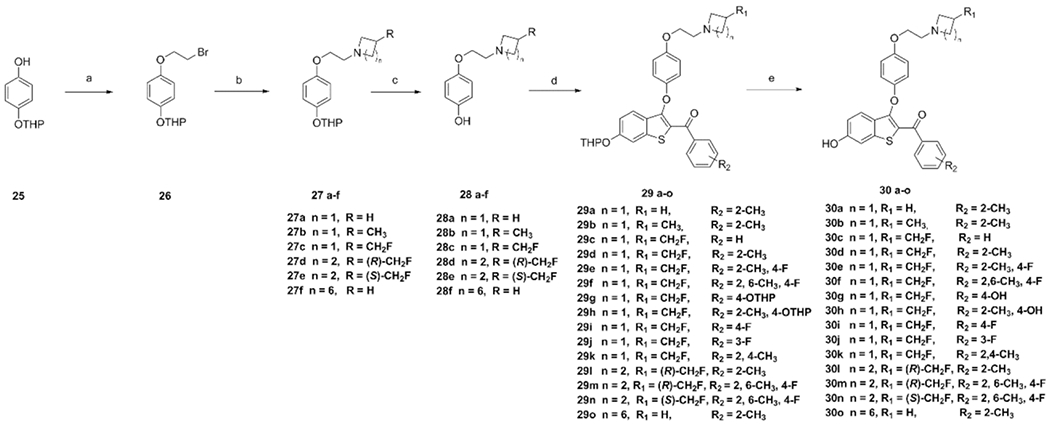
aReagents and conditions: (a) 1,2-dibromoethane, KOH, THF, reflux, 50%. (b) Azetidine hydrochloride, 3-methylazetidine hydrochloride, 20, 24, piperidine, NaH, THF, 0–60 °C, 55%, (c) p-TsOH, MeOH, rt, 60%. (d) 16a-i, Cs2CO3, DMF, 90 °C, 55–75%. (e) p-TsOH, MeOH, rt, 60–80%.
