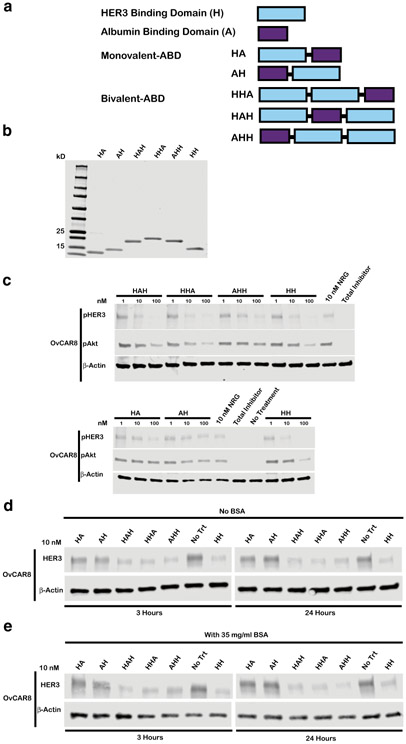
Fig. 4.
Arrangement of HER3 affibody domains and albumin-binding domains in a fusion protein has minimal impact on bioactivity. a Schematic representing monovalent and bivalent albumin-binding domain fusion constructs where “H” denotes HER3 binding domain and “A” denotes albumin-binding domain b Purified protein products are displayed on a Coomassie stained gel loaded at 10 μg/lane. Monovalent ABDs HA and AH have respective theoretical molecular weights of 12.9 and 13.0 kDa, bivalent ABDs HHA, HAH, and AHH have MW of 19.7, 19.7, and 19.8 kDa respectively, and HH-bivalent HER3 affibody positive control lacking ABD has MW of 14.4 kDa. c OvCAR8 cells were treated with the indicated concentration affibody construct, 100 nM pan-HER kinase inhibitor (total inhibitor), or media alone (no treatment) for 30 min, then stimulated with 10 nM NRG for 10 min (except for no treatment negative control), lysed and probed for pHER3, pAkt, and β-actin by immunoblot. In the d absence of bovine serum albumin (BSA) or e presence of 35 mg/ml BSA, OvCAR8 cells were treated with 10 nM of the indicated affibody construct or media alone (no treatment), lysed at the indicated time points and probed by immunoblot for HER3 and β-actin. All multivalent ligand concentrations refer to the concentration of individual affibody domains. Results shown are representative of three independent experiments. All blots were cropped; blots for pHER3 and pAkt are presented at different exposures for clarity