Figure 3.
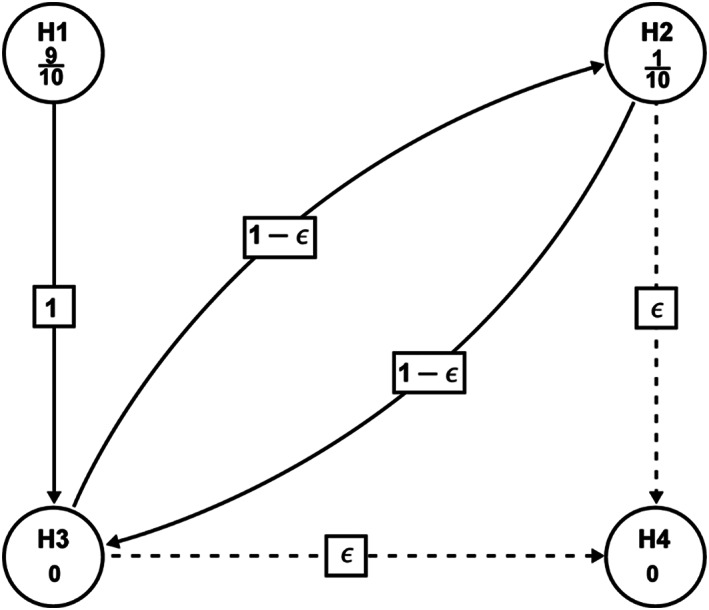
Graphical illustration of the sequentially rejective testing procedure. H1: Sacubitril/valsartan is no better than the comparator in change from baseline in log‐transformed NT‐proBNP at Week 12 (Primary). H2: Sacubitril/Valsartan is no better than the comparator in change from baseline in 6 min walking distance (6MWD) at Week 24. (Primary). H3: Sacubitril/valsartan is no better than the comparator in change from baseline in Kansas City Cardiomyopathy Questionnaire (KCCQ) Clinical Summary Score (CSS) (mean score) at Week 24. H4: Sacubitril/valsartan is no better than the comparator in NYHA class change from baseline at Week 24. In order to control the family‐wise type‐I error rate at the one‐sided 0.025 significance level, a sequentially rejective multiple testing procedure will be employed, whereby H1 and H2 will be tested first at initially assigned level of one‐sided (9/10) × α = 0.0225 and one‐sided (1/10) × α = 0.0025, accordingly. If H1 and/or H2 are rejected, the alpha for the rejected null hypotheses will be propagated to H3, such that, H3 will be tested at the updated alpha level (one‐sided 0.025 if both H1 and H2 are rejected; one‐sided 0.0225 if H1 is rejected but H2 is not rejected; one‐sided 0.0025 if H2 is rejected but H1 is not rejected); if H3 is rejected, the alpha will be propagated to H2 or H4 based on the initial step rejection status
