Abstract
Aims
Sufficient myocardial recovery with the subsequent explantation of a left ventricular assist device (LVAD) occurs in approximately 1–2% of the cases. However, follow‐up data about this condition are scarcely available in the literature. This study aimed to report the long‐term outcomes and clinical management following LVAD explantation.
Methods and results
An analysis of the European Registry for Patients with Mechanical Circulatory Support was performed to identify all adult patients with myocardial recovery and successful explantation. Pre‐implant characteristics were retrieved and compared with the non‐recovery patients. The follow‐up data after explantation were collected via a questionnaire. A Kaplan–Meier analysis for freedom of the composite endpoint of death, heart transplantation, LVAD reimplantion, or heart failure (HF) relapse was conducted. A total of 45 (1.4%) cases with myocardial recovery resulting in successful LVAD explantation were identified. Compared with those who did not experience myocardial recovery, the explanted patients were younger (44 vs. 56 years, P < 0.001), had a shorter duration of cardiac disease (P < 0.001), and were less likely to have ischaemic cardiomyopathy (9% vs. 41.8%, P < 0.001). Follow‐up after explantation could be acquired in 28 (62%) cases. The median age at LVAD implantation was 43 years (inter‐quartile range: 29–52), and 23 (82%) were male. Baseline left ventricular ejection fraction was 18% (inter‐quartile range: 10–20%), and 60.7% of the patients had Interagency Registry for Mechanically Assisted Circulatory Support Profile 1 or 2. Aetiologies of HF were dilated cardiomyopathy in 36%, myocarditis in 32%, and ischaemic in 14% of the patients, and 18% had miscellaneous aetiologies. The devices implanted were HeartMate II in 14 (50%), HVAD in 11 (39%), HeartMate 3 in 2 (7%), and 1 unknown with a median duration of support of 410 days (range: 59–1286). The median follow‐up after explantation was 26 months (range 0.3–73 months), and 82% of the patients were in New York Heart Association Class I or II. Beta‐blockers were prescribed to 85%, angiotensin‐converting enzyme inhibitors to 71%, and loop diuretics to 50% of the patients, respectively. Freedom from the composite endpoint was 100% after 30 days and 88% after 2 years.
Conclusions
The survival after LVAD explantation is excellent without the need for heart transplantation or LVAD reimplantation. Only a minority of the patients suffer from a relapse of significant HF.
Keywords: Mechanical circulatory support, Left ventricular assist device, Myocardial recovery, Explantation, Survival
1. Introduction
Continuous flow left ventricular assist devices (cf‐LVADs) have become an important modality in the treatment of end‐stage heart failure (HF) as a bridge to transplantation (BTT), bridge to candidacy, or as destination therapy. This has led to a significant improvement of the quality of life and overall survival of patients once all other therapeutic options have been exhausted.1
A small percentage of these patients experience significant myocardial recovery under LVAD support and can therefore undergo LVAD explantation, defined as actual bridge to recovery (BTR).1 The eighth Interagency Registry for Mechanically Assisted Circulatory Support (INTERMACS) annual report and the second European Registry for Patients with Mechanical Circulatory Support (EUROMACS) report, reported that 1–2% of the patients implanted with cf‐LVADs recovered, allowing successful LVAD explantation.1, 2 Similarly, in a pooled HeartMate II cohort with 1108 patients enrolled, the rate of myocardial recovery was 1.8%.3
Several case series and small cohort studies report patients having sufficient recovery of left ventricle (LV) function that allowed LVAD explantation. These studies show higher rates of myocardial recovery after LVAD explantation and survival ranging from 78.3% to 100%, with varying rates of HF recurrence.4, 5, 6, 7, 8
These studies report encouraging survival outcomes; however, much remains unknown concerning adverse events (AEs) and HF management after explantation and most studies are based on single‐centre experiences. Little is known about long‐term outcomes and AEs, such as ventricular tachycardia or thromboembolic complications, given the fact that the inflow cannula is not (always) extracted and data about its management are usually lacking. Furthermore, the follow‐up of these patients is limited, and details on specific medication are lacking and not consistent because of a small number of patients. There is no evidence‐based knowledge regarding the long‐term treatment of these patients in case of recurrence of HF or other complications. Such inconsistencies prompt a need for a complete long‐term follow‐up in these patients, with a special emphasis on chronic medication, AEs, and longer‐term survival after successful LVAD explantation.
1.1. Objectives
The aim of the study is to evaluate long‐term outcomes and patient management after a successful LVAD termination due to the recovery, including survival, complications, relapse of HF, and specific medical treatments.
2. Methods
2.1. Study design
A retrospective study was conducted in all patients in whom an LVAD was successfully explanted after myocardial recovery as registered in the EUROMACS.2, 9 Inclusion criteria were successful explantation of a cf‐LVAD (as a stand‐alone VAD system, not right VAD) as captured in the follow‐up of EUROMACS. Exclusion criteria included an explantation due to any other reason than myocardial recovery (e.g. infection and device malfunction) or patients aged <18 years.
2.2. Data collection
From January 2011 until March 2018, a total of 45 patients in the EUROMACS registry were identified as being successfully explanted after myocardial recovery, from now on named recovery patients. Baseline characteristics before LVAD implantation including age, sex, aetiology of HF, pre‐operative condition and co‐morbidities, electrocardiogram, echocardiogram, and blood chemistry values were collected for adult patients in EUROMACS. Furthermore, perioperative data on device strategy, device type, concomitant surgical procedures, and cardiopulmonary bypass (CPB) time, time in operating room, intensive care unit, and hospital stay were retrieved. Finally, time on LVAD and type and number of AEs and hospitalizations were collected from the follow‐up.
Subsequently, a detailed questionnaire was sent to involved centres to attain the follow‐up of these patients after LVAD explantation. These data are currently not captured in the EUROMACS registry. Because data entry into EUROMACS is anonymized for external reviewers, the executive director (T. M. M. H. d. B.) approached the centres asking to provide the follow‐up of these patients. Data collected included the primary outcome: survival, heart transplantation (HTx), reimplantation of LVAD, and data on re‐hospitalizations due to HF. Secondary outcomes consisted of the following parameters: presence of the inflow cannula, the occurrence of cerebrovascular accidents, New York Heart Association (NYHA) class at last follow‐up, oral anticoagulation, and HF medication. Finally, data of electrocardiogram, echocardiography, and blood chemistry values were requested.
2.3. Statistical analysis
Continuous parameters are expressed as mean and confidence interval or median and range or inter‐quartile range. Categorical parameters are expressed as number and percentage. For categorical parameters, χ 2 test and Fisher's exact test were applied as appropriate. For continuous parameters, Student's t‐test and Wilcoxon rank‐sum test were used. A comparison of baseline characteristics was performed to assess differences in patients with VAD explantation and without VAD explantation. Furthermore, baseline characteristics of patients whose LVAD was explanted with and without follow‐up were compared to assess a potential reporting bias. Finally, a Kaplan–Meier curve was constructed to evaluate freedom of the composite endpoint of death, HTx, LVAD reimplantation, or relapse of HF ≥ NYHA III after LVAD explantation. Statistical analysis was performed using SPSS, Version 25.0 for Windows (IBM Inc., Armonk, NY, USA).
3. Results
3.1. Baseline characteristics
A total of 45 patients in whom the LVAD was explanted because of myocardial recovery were identified in the EUROMACS registry, representing 1.4% of the patients registered at the time. A complete follow‐up of 28 (62.2%) recovery patients after explantation was obtained. In these patients, median age at implantation was 43 years (range 29–52) and 23 patients (82.1%) were male (Table 1 A). Predominant aetiologies of HF were myocarditis in nine (32.1%) patients and dilated cardiomyopathy in 10 (35.7%) patients. Most patients had a short history of cardiac disease, with 14 (50%) patients having had their first cardiac diagnosis less than 1 month prior to the LVAD implantation and 20 (71.4%) within 1 year prior to the implantation. Patients were almost evenly distributed over INTERMACS Patient Profiles I to III, only three patients had INTERMACS Patient Profile IV–V. Median LVEF was 18% (inter‐quartile range: 10–20%), whereas five patients exhibited greater than or equal to moderate mitral regurgitation. At the time of implant, 23 (82.1%) patients had inotropic support and 14 patients (50%) experienced any form of mechanical circulatory support by either extracorporeal life support or an intra‐aortic balloon pump. There were, apart from mitral regurgitation, no significant differences between patients with or without an obtained follow‐up after LVAD explantation (Table 1 A).
Table 1.
A: Baseline characteristics of all patients with left ventricular assist device explantation due to myocardial recovery with and without follow‐up after explantation
| With follow‐up (28) | No follow‐up (17) | P‐value | |
|---|---|---|---|
| Age (years) | 43 (29–52) | 53 (41–65) | 0.053 |
| Male | 23 (82.1) | 13 (76.5) | 0.711 |
| BMI(kg/m2) | 26.9 [25.1–28.6] | 25.7 [23.2–28.2] | 0.182 |
| BSA(m2) | 2.02 [1.92–2.12] | 1.98 [1.87–2.10] | 0.395 |
| Aetiology | 0.579 | ||
| Myocarditis | 9 (32.1) | 3 (17.6) | |
| Dilated cardiomyopathy | 10 (35.7) | 4 (23.5) | |
| Ischaemic cardiomyopathy | 4 (14.3) | 5 (27.1) | |
| Peripartum | 1 (3.6) | 1 (5.9) | |
| Valvular heart disease | 2 (7.1) | 2 (11.8) | |
| Hypertrophic cardiomyopathy | 0 (0) | 1 (5.9) | |
| Toxic | 1 (3.6) | 0 (0) | |
| Restrictive cardiomyopathy | 0 (0) | 0 (0) | |
| Congenital heart disease | 0 (0) | 0 (0) | |
| Other/unknown | 1 (3.6) | 1 (5.9) | |
| Time since first cardiac diagnosis | 0.453 | ||
| <1 month | 14 (50) | 6 (35.3) | |
| 1 month–1 year | 6 (21.4) | 5 (29.4) | |
| 1 year or more | 4 (14.3) | 5 (29.4) | |
| Unknown | 4 (14.3) | 1 (5.9) | |
| Current ICD in place | 2 (7.1) | 2 (11.8) | 1.000 |
| INTERMACS profiles | 0.918 | ||
| INTERMACS 1 | 8 (28.6) | 6 (35.3) | |
| INTERMACS 2 | 9 (32.1) | 6 (35.3) | |
| INTERMACS 3 | 8 (28.6) | 3 (17.6) | |
| INTERMACS 4–5 | 3 (10.7) | 2 (11.8) | |
| INTERMACS 6–7 | 0 (0) | 0 (0) | |
| Echocardiography | |||
| LVEF (%) | 18 (10–20) | 15 (15–20) | 0.612 |
| LVEDD (mm) | 68 (63–70) | 66 (62–73) | 0.771 |
| Aortic regurgitation ≥ moderate | 1 | 0 | 1.000 |
| Mitral regurgitation ≥ moderate | 4 | 9 | 0.038 |
| ECG rhythm | 0.389 | ||
| Sinus | 18 (64.3) | 14 (82.4) | |
| Atrial fibrillation/flutter | 5 (17.9) | 3 (17.6) | |
| Paced | 1 (3.6) | 0 (0) | |
| Other/unknown | 4 (14.3) | 0 (0) | |
| Heart rate (b.p.m.) | 97 (89–121) | 98 (74–111) | 0.455 |
| Blood pressure (mmHg) | |||
| Systolic | 105 (92–115) | 106 (94–114) | 0.919 |
| Diastolic | 61 (60–70) | 67 (50–70) | 0.942 |
| Mean arterial pressure | 74 (72–84) | 78 (70–82) | 0.965 |
| Diabetes | 2 (7.1) | 0 (0) | 0.519 |
| Inotropic support | |||
| Intravenous inotropes | 23 (82.1) | 14 (82.4) | 0.333 |
| 1–2 inotropes | 18 (64.3) | 8 (47.1) | |
| ≥3 inotropes | 5 (17.9) | 6 (35.3) | |
| IABP | 4 (14.3) | 3 (17.6) | 1.000 |
| ECLS | 10 (35.7) | 7 (41.2) | 0.715 |
| Mechanical ventilation | 8 (28.6) | 6 (35.3) | 0.637 |
| Blood chemistry | |||
| Creatinine (μmol/L) | 105 (79–114) | 114 (91–141) | 0.142 |
| ALAT (U/L) | 76 (39–177) | 46 (34–520) | 0.892 |
| ASAT (U/L) | 180 (42–592) | 73 (27–184) | 0.147 |
| LDH (U/L) | 469 (308–1189) | 407 (338–992) | 0.859 |
| Total bilirubin (mg/dL) | 1.5 (0.8–2.5) | 1.5 (0.8–2.1) | 0.525 |
| Haemoglobin (g/dL) | 11.8 (10.2–13.4) | 10.9 (10.4–13.7) | 0.971 |
| White blood cell count (× 109/L) | 10.6 (9.3–14.3) | 10.7 (8.7–13.4) | 0.819 |
| Thrombocytes (× 109/L) | 164 (75–241) | 191 (104–266) | 0.479 |
ALAT, alanine aminotransaminase; ASAT, aspartate transaminase; BMI, body mass index; BSA, body surface area; ECG, electrocardiogram; ECLS, extracorporeal life support; IABP, intra‐aortic balloon pump; ICD, implantable cardioverter‐defibrillator; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LDH, lactate dehydrogenase; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction.
Values are median (inter‐quartile range), mean [confidence interval], or n (%).
Table 1.
B: Baseline characteristics of all patients with and without left ventricular assist device explantation due to myocardial recovery
| Explanted | Not explanted | P‐value | |
|---|---|---|---|
| Age (years) | 44 (32–54) | 56 (47–62) | <0.001 |
| Male | 36 (80.0) | 2568 (83.6) | 0.542 |
| BMI(kg/m2) | 26.4 [25.0–27.8] | 26.1 [26.0–26.3] | 0.691 |
| BSA(m2) | 2.00 [1.93–2.08] | 1.97 [1.96–1.98] | 0.313 |
| Aetiology | <0.001 | ||
| Myocarditis | 12 (26.7) | 125 (4.1) | |
| Dilated cardiomyopathy | 14 (31.1) | 995 (32.4) | |
| Ischaemic cardiomyopathy | 9 (20) | 1285 (41.8) | |
| Peripartum | 2 (4.4) | 14 (0.5) | |
| Valvular heart disease | 4 (8.9) | 45 (1.5) | |
| Hypertrophic cardiomyopathy | 1 (2.2) | 30 (1.0) | |
| Toxic | 1 (2.2) | 51 (1.7) | |
| Restrictive cardiomyopathy | 0 (0) | 20 (0.7) | |
| Congenital heart disease | 0 (0) | 28 (0.9) | |
| Other/unknown | 2 (4.4) | 480 (15.6) | |
| Time since first cardiac diagnosis | <0.001 | ||
| <1 month | 20 (44.4) | 302 (11.1) | |
| 1 month–1 year | 11 (24.4) | 356 (13.1) | |
| 1 year or more | 9 (20) | 1857 (68.4) | |
| Unknown | 5 (11.1) | 198 (7.3) | |
| Current ICD in place | 1633 (61.4) | 4 (8.9) | <0.001 |
| INTERMACS profiles | 0.01 | ||
| INTERMACS 1 | 14 (31.1) | 412 (13.4) | |
| INTERMACS 2 | 15 (33.3) | 996 (32.4) | |
| INTERMACS 3 | 11 (24.4) | 781 (25.4) | |
| INTERMACS 4–5 | 5 (11.1) | 637 (21.6) | |
| INTERMACS 6–7 | 0 (0.0) | 123 (4.2) | |
| Echocardiography | |||
| LVEF (%) | 17 (15–19) | 19 (19–19) | 0.125 |
| LVEDD (mm) | 65.6 (62.2–69.0) | 71.3 (70.1–72.6) | 0.270 |
| Aortic regurgitation greater than or equal to moderate | 1 (3.4) | 92 (3.8) | 1.000 |
| Mitral regurgitation greater than or equal to moderate | 13 (38.2) | 1221 (50.9) | 0.141 |
| ECG rhythm | 0.005 | ||
| Sinus | 32 (71.1) | 1359 (51.5) | |
| Atrial fibrillation/flutter | 8 (17.8) | 424 (16.1) | |
| Paced | 1 (2.2) | 663 (25.1) | |
| Other/unknown | 4 (8.9) | 192 (7.3) | |
| Heart rate (b.p.m.) | 100 (92–107) | 87 (86–87) | <0.001 |
| Blood pressure (mmHg) | |||
| Systolic | 106 (100–112) | 100 (100–102) | 0.048 |
| Diastolic | 64 (59–69) | 65 (64–65) | 0.725 |
| Mean arterial pressure | 78 (73–83) | 77 (76–77) | 0.519 |
| Diabetes | 2 (4.4) | 2105 (73.5) | 0.001 |
| Inotropic support | |||
| Intravenous inotropes | 37 (82.2) | 2495 (89) | 0.547 |
| 1–2 inotropes | 26 (57.8) | 2149 (76.7) | |
| ≥3 inotropes | 11 (24.4) | 346 (12.3) | |
| IABP | 7 (15.9) | 297 (11.3) | 0.342 |
| ECLS | 17 (37.8) | 303 (10.5) | <0.001 |
| Mechanical ventilation | 14 (31.1) | 406 (15.4) | 0.004 |
| Blood chemistry | |||
| Creatinine (μmol/L) | 111 (83–123) | 111 (85–150) | 0.465 |
| ALAT (U/L) | 29 (54–177) | 29 (18–70) | 0.001 |
| ASAT (U/L) | 135 (31–410) | 33 (23–74) | <0.001 |
| LDH (U/L) | 434 (314–1173) | 308 (238–452) | <0.001 |
| Total bilirubin (mg/dL) | 1.78 (0.78–2.26) | 1.29 (0.80–2.10) | 0.591 |
| Haemoglobin (g/dL) | 11.9 (10.3–13.6) | 11.8 (10.2–13.5) | 0.844 |
| White blood cell count (× 109/L) | 10.7 (8.9–14.0) | 8.4 (6.7–11.0) | <0.001 |
| Thrombocytes (× 109/L) | 173 (80–247) | 199 (150–250) | 0.030 |
ALAT, alanine aminotransaminase; ASAT, aspartate transaminase; BMI, body mass index; BSA, body surface area; ECG, electrocardiogram; ECLS, extracorporeal life support; IABP, intra‐aortic balloon pump; ICD, implantable cardioverter‐defibrillator; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; LDH, lactate dehydrogenase; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction.
Values are median (inter‐quartile range), mean [confidence interval], or n (%).
A comparison between patients whose LVAD was explanted and patients with another outcome (ongoing, HTx or death) reveals that recovery patients were significantly younger (45 vs. 53.5 years; P < 0.001), had a shorter duration of cardiac disease (P < 0.001) and less implantable cardioverter‐defibrillators implanted (8.9% vs. 61.4%; P < 0.001), and were more often in INTERMACS Patient Profile 1 (P = 0.01; Table 1B). Furthermore, the predominant aetiologies of HF were myocarditis and dilated cardiomyopathy for recovery patients, while ischaemic cardiomyopathy was the main cause of HF in the non‐recovery group.
3.2. Perioperative characteristics
The indication designation was BTT in 22 (78.5%), destination therapy in 2 (7.1%), and rescue therapy in 4 (14.3%) (Table 2). Implanted devices included the HeartMate II (n = 14) (Abbott, Lake Bluff, IL, USA), HeartWare HVAD (n = 11) (Medtronic, Minneapolis, MN, USA), HeartMate 3 (n = 2) (Abbott, Lake Bluff, IL, USA), and one unknown device. Concomitant cardiac surgery was performed in five patients: three patent foramen ovale repairs, one tricuspid valve repair, and one aortic valve replacement. Two patients received a temporary right VAD. Median time in the operating room was 208 min (range: 130–683), with a median CPB time of 75 min (95–147). Post‐operative intensive care unit stay ranged from 4 to 147 days with a median of 17 days and a median hospital stay of 30 days (17–165). Patients with a follow‐up after LVAD explantation had significantly different device strategies (P < 0.001) (less BTR and more BTT patients) and a shorter duration of CPB (P = 0.034) compared with patients without a follow‐up.
Table 2.
Implantation and post‐implantation characteristics for recovery patients
| With follow‐up (28) | No follow‐up (17) | P‐value | |
|---|---|---|---|
| Device strategy | <0.001 | ||
| BTT | 22 (78.5%) | 7 (36.9%) | |
| DT | 2 (7.1%) | 2 (10.5%) | |
| Rescue therapy | 4 (14.3%) | 2 (10.5%) | |
| Bridge to recovery | 0 (0%) | 8 (42.1%) | |
| Device type | 0.204 | ||
| HeartMate II | 14 (50%) | 6 (31.6%) | |
| HVAD | 11 (39.3%) | 9 (47.4%) | |
| HeartMate 3 | 2 (7.1%) | 0 (0%) | |
| PVAD | 0 (0%) | 2 (10.5%) | |
| Other/unknown | 1 (3.6%) | 2 (10.5%) | |
| Concomitant cardiac procedures | |||
| PFO/ASD closure | 3 (10.7%) | 2 (10.5%) | |
| Tricuspid repair | 1 (3.6%) | 4 (21.1%) | |
| Tricuspid replacement | 1 (5.3%) | ||
| Aortic repair | 1 (5.3%) | ||
| Aortic valve replacement | 1 (3.6%) | ||
| Mitral repair | 1 (5.3%) | ||
| CABG | 1 (5.3%) | ||
| Concomitant temporary RVAD implantation | 2 (7.1%) | 2 (10.5%) | 1.000 |
| Time in OR (min) | 208 (130–683) | 276 (95–375) | 0.600 |
| Cardiopulmonary bypass time (min) | 75 (95–147) | 124 (50–235) | 0.034 |
| ICU stay (days) | 17 (4–147) | 27 (2–66) | 0.377 |
| Hospital stay (days) | 30 (17–165) | 39 (14–144) | 0.328 |
ASD, atrial septal defect; BTT, bridge to transplantation; CABG, coronary artery bypass grafting; DT, destination therapy; ICU, intensive care unit; OR, operating room; PFO, patent foramen ovale; RVAD, right ventricular assist device.
Values are median (range) or n (%).
3.3. Outcomes during ventricular assist device support
The median support time of the patients was 410 days (59–1286). Within this time frame, the following key AEs were captured: major infection, major bleeding and device malfunction, and haemolysis (Table 3). Eight (28.6%) patients remained free of any AEs during LVAD support, while 10 patients (35.7%) encountered three or more AEs. Forty‐eight (73.8%) captured AEs required a hospitalization. There were no significant differences to the patients with missing follow‐up after explantation.
Table 3.
Time on support and AEs during mechanical circulatory support for recovery patients
| With follow‐up (28) | No follow‐up (17) | P‐value | |
|---|---|---|---|
| Time on support (days) | 410 (59–1286) | 231 (10–1425) | 0.06 |
| Type of AEs | |||
| Major infection | 21 (32.3%) | 4 (14.3%) | |
| Major bleeding | 8 (12.3%) | 1 (3.6%) | |
| Device malfunction | 6 (9.2%) | 5 (17.9%) | |
| Haemolysis | 4 (6.2%) | 2 (7.1%) | |
| Cardiac arrhythmia | 3 (4.6%) | 1 (3.6%) | |
| Stroke | 2 (3.1%) | 3 (10.7%) | |
| Renal dysfunction | 2 (3.1%) | 4 (14.3%) | |
| Right heart failure | 0 (0%) | 1 (3.6%) | |
| Other | 19 (29.2%) | 7 (25%) | |
| Number of AEs per patient | 0.889 | ||
| 0 | 8 (28.6%) | 5 (29.4%) | |
| 1–2 | 10 (35.7%) | 8 (47.1%) | |
| 3–4 | 7 (25.0%) | 3 (17.6%) | |
| ≥5 | 3 (10.7%) | 1 (5.9%) | |
| Hospitalizations required for AE | 48 (73.8%) | 26 (92.9%) | |
| Hospitalizations per patient | 1.000 | ||
| 0 | 10 (35.7%) | 6 (35.3%) | |
| 1–2 | 11 (39.3%) | 7 (41.2%) | |
| 3–4 | 5 (17.9%) | 3 (17.6%) | |
| ≥5 | 2 (7.1%) | 1 (5.9%) |
AE, adverse event.
Values are median (range) or n (%).
3.4. Outcomes after left ventricular assist device explantation
Median follow‐up time after LVAD explantation is 26 months (0.3–73). Freedom from death, LVAD reimplantation, HTx, and relapse of HF ≥ NYHA III was 100% at 30 days and 88% at 24 months after explantation (Figure 1 and Table 4). Two patients encountered an HF relapse, which is in part attributable to new onset of degenerative mitral regurgitation in one patient. One patient required reimplantation of an LVAD after 32 days. Finally, one patient died 302 days after LVAD explantation due to sepsis. Until 48 months, this percentage remained unchanged (88%); however, follow‐up was only available for six patients for this duration of follow‐up.
Figure 1.
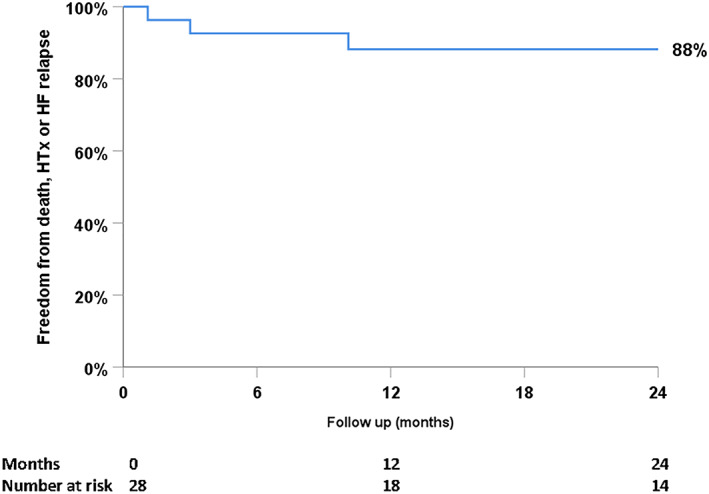
Freedom of death, left ventricular assist device reimplantation, heart transplantation (HTx), and significant heart failure (HF) relapse after left ventricular assist device explantation.
Table 4.
Long‐term outcome post‐LVAD explant for recovery patients
| Follow‐up time (months) | 26 (0.3–73) |
| Primary outcome | |
| Ongoing after explant | 26 (92.8%) |
| HF recurrence | 3 (10.7%) |
| LVAD reimplantation | 1 (3.6%) |
| Death | 1 (3.6%) |
| BMI(kg/m2) | 27.6 [25.4–29.7] |
| Blood pressure (mmHg) | |
| Systolic | 113 (88–160) |
| Diastolic | 77 (51–98) |
| Mean arterial pressure | 90 (68–113) |
| Echocardiography data | |
| LVEF (%) | 40 (15–60) |
| LVEDD (mm) | 54 (41–74) |
| LVESD (mm) | 43 (27–63) |
| MR grade ≥3 | 2 |
| ECG | |
| Heart rate (b.p.m.) | 73 (48–105) |
| Rhythm | |
| Sinus | 21 (75%) |
| Atrial fibrillation/flutter | 3 (10.7%) |
| Other | 4 (14.3%) |
| QRS width (ms) | 98 (74–188) |
| QTc duration (ms) | 435 (374–593) |
| Blood chemistry | |
| Creatinine (μmol/L) | 97 (62–248) |
| Bilirubin (mg/dL) | 0.6 (0.3–2.8) |
| Functional status at last follow‐up | |
| NYHA Class I | 7 (25%) |
| NYHA Class II | 16 (57.2%) |
| NYHA Class III | 3 (10.7%) |
| Unknown | 2 (7.1%) |
| HF medication | |
| Beta‐blockers | 24 (85.7%) |
| ACE inhibitors | 20 (71.4%) |
| Loop diuretics | 14 (50%) |
| Inflow cannula in situ | 3 (10.7%) |
| Anticoagulation/anti‐platelet therapy | 20 (71.4%) |
| Acetylsalicylic acid | 12 (42.9%) |
| Vitamin K antagonist | 11 (39.3%) |
| Both | 4 (14.3%) |
| None | 8 (29%) |
| Number of patients with CVA | 0 (0%) |
ACE, angiotensin‐converting enzyme; BMI, body mass index; CVA, cerebrovascular accident; ECG, electrocardiogram; HF, heart failure; LVAD, left ventricular assist device; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVESD, left ventricular end‐systolic diameter; MR, mitral regurgitation; NYHA, New York Heart Association.
Values are median (range), mean [standard deviation], or n (%).
Median LVEF at last follow‐up is 40% (15–60), with a median left ventricular end‐diastolic and end‐systolic diameter of 54 mm (41–74) and 43 mm (27–63), respectively. Patient's HF symptoms are predominantly NYHA I and II (82.2%) with three patients suffering significant HF symptoms (NYHA III). All patients were on at least one type of HF medication: with beta‐blockers in 24/28 (85.7%) and angiotensin‐converting enzyme inhibitors in 20/28 patients (71.4%) being used most frequently.
The inflow cannula remained in situ after explantation in three patients (11%). Of these patients, two were on both warfarin and aspirin and one patient only used warfarin. The inflow cannula was not in situ in the 25 other patients, two of them used both warfarin and aspirin. Long‐term anticoagulation treatment was aspirin in 43% of the patients, while in 39%, warfarin was used. Four patients were prescribed with both aspirin and warfarin. In eight (29%) patients, no anti‐platelet or anticoagulation therapy was used. No cerebrovascular accident was reported in any patient.
4. Discussion
This study provides a multi‐centre, mid‐ to long‐term follow‐up of patients whose LVAD was explanted because of myocardial recovery. The mid‐ to long‐term outcomes appear to be encouraging with 88% of the patients surviving without HTx, LVAD reimplantation, or relapse of HF at 24 months of follow‐up. Furthermore, the majority of patients suffered from mild HF‐symptoms only (NYHA Class I–II). The number of patients with sufficient myocardial recovery for LVAD explantation is comparable with other registries such as INTERMACS, which reported a successful weaning rate of approximately 1% in the latest annual report.10, 11
A comprehensive review of single‐centre studies reporting on myocardial recovery allowing LVAD explantation found weaning rates ranging from 4.5% to 63% and thus contrasts with lower recovery rates reported in the INTERMACS or EUROMACS registries.12 Some of these studies have reported higher rates of successful weaning. Interestingly, some showed a successful explantation rate of 12 in 19 (63%) patients supported by a HeartMate II.13 However, this population was young (mean age 35.2 years) and patients with ischaemic heart disease were excluded. Moreover, they received aggressive pharmacotherapy with maximum HF medication combined with clenbuterol (β2‐agonist).
These high rates of recovery may partly be explained because of the commitment of some centres resulting in specific clinical and scientific focus on the recovery. This might result in advanced, aggressive strategies to identify potential patients eligible for LVAD explantation and thus treating those patients with targeted and strictly regulated HF medication. Furthermore, studies that included patients with non‐ischaemic heart disease as aetiology of HF and patients with recent onset of HF tend to have higher rates of successful myocardial recovery. These observations correspond well with our study in which the majority of patients had their first cardiac disease diagnosis less than 1 year ago and ischaemic cardiomyopathy represented an uncommon aetiology of HF. Indeed, the baseline characteristics that are significantly different in patients with LVAD explantation are very similar to the variables used in the INTERMACS Cardiac Recovery Score of Wever‐Pinzon et al.11
In the literature, several case series and small cohort studies report on patients having recovery of left ventricle (LV) function that allowed LVAD explantation. Studies such as Dandel et al.4 report that LVAD (Novacor) explantation was successful in 32 of 131 patients with idiopathic dilated cardiomyopathy, with a 5 year survival of 78.3% and 31.1% HF recurrence rate in 3 years, after being supported with an LVAD for a mean duration of 4.6 months (SD ± 4.4). Birks et al.5 reported that out of 15 patients with severe HF due to non‐ischaemic cardiomyopathy, 11 had LVAD explantation after a mean of 320 days of LVAD support; 88.9% of the surviving patients were free from HF at 4 years after explantation. Another study showed that survival after explantation of HeartMate II was 83.3% after 3 year follow‐up.13 A study of 14 patients revealed that after a mean follow‐up time of 3.6 years (±1.9) after explantation, no patient had died and had a functional NYHA class of I.6 Frazier et al.7 achieved explantation in 27 patients out 657 patients supported by an LVAD, with 25 of them surviving after a mean follow‐up of 3.2 years (±2.6) all of them with NYHA Class I with medical therapy. In comparison, we showed a similar excellent survival of 88% without HTx, LVAD reimplantation or significant HF relapse in a European multi‐centre registry, with the majority of patients only suffering from mild HF symptoms after explantation. This highlights that, in carefully selected patients, excellent results can be achieved after LVAD explantation and are not restricted to one centre.
4.1. Pathophysiology and clinical implications of left ventricular recovery
The pathophysiology of HF is complex and multifactorial. Systolic HF is accompanied by LV remodelling, which is characterized by three categories of changes in the heart: myocyte defects, myocardial defects, and abnormal LV geometry.14 In contrast, to achieve myocardial recovery, the heart has to undergo cardiac reverse remodelling. Support by cf‐LVADs results in left ventricular pressure and volume unloading as well as increased cardiac output15, 16 and has shown to promote certain forms of reverse remodelling17: reduction of cardiac myocyte hypertrophy,18, 19 changes in gene expression,20, 21 and normalization of β‐adrenergic receptor and inotropic responsiveness.22 Concerning restoration of the extracellular matrix, conflicting studies exist, with some studies reporting an increase in total extracellular matrix collagen,23, 24 while others report a decrease.25 Finally, studies have shown an improvement in cardiac myocyte contractility after LVAD implantation.26, 27 There are some excellent reviews covering this topic in much more detail.14, 28 On the clinical side, the criteria used for the decision of LVAD explantation differ between centres.8, 13, 29 Clinical parameters that indicate LV remodelling and might indicate myocardial recovery often include an increase in LVEF, decreases in end‐diastolic left ventricular diameter, (partial) reversal of functional mitral regurgitation, normalization of cardiac filling pressures, and cardiac sinus rhythm with a normal heart rate. Unfortunately, studies linking pathophysiological findings with these clinical outcomes are scarce.
4.2. Future perspectives
A combination of clinical and biological findings for patients undergoing LVAD implantation is currently lacking robust data with both studies conducted separately of each other. Preferably, one would collect histological/biological data and clinical data before LVAD implantation, during LVAD support, and after explantation (if applicable). This holistic approach would provide much needed insight in the changes that are induced by VAD therapy and would enable us to link and understand pathophysiological changes to clinical changes and vice versa.
Finally, because the number of patients that have sufficient myocardial recovery to enable LVAD explantation is limited, it is critical that researchers and clinicians cooperate in large registries such as EUROMACS and INTERMACS, by adding data fields for centres willing to capture data on the follow‐up of these patients. As a consequence, the EUROMACS board set out the goal that follow‐up of after explantation of VAD devices due to recovery should also be captured in the near future.
4.3. Limitations
This study has certain limitations that should be considered while interpreting the results. Data were gathered retrospectively, and the number of data fields captured is limited. Furthermore, it is possible that not all patients whose LVAD was explanted because of recovery were captured in the EUROMACS registry. It is also possible that the follow‐up of patients who actually have been explanted because of recovery has not been registered yet. This might result in a relative underestimation of the number of patients with myocardial recovery; however, the EUROMACS registry regularly checks and audits participating centres for data quality and completion. Finally, we only received follow‐up data on 62% of those patients weaned from LVAD support, which may constitute a potential selection bias resulting in favourable outcomes. However, the baseline characteristics and follow‐up during LVAD support of patients with and without follow‐up were, apart from one variable, not significantly different (Table 1 A).
5. Conclusions
To our knowledge, this is one of the first multi‐centre studies to review midterm to long‐term follow‐up after LVAD explantation due to myocardial recovery. Although LVAD explantation remains rare, outcomes after explantation are excellent with a majority of patients ongoing without HTx or LVAD reimplantation while having limited HF symptoms only. Large, prospective registries and/or studies are required to generate pertinent data in order to better understand this challenging population.
Conflict of interest
None declared.
Funding
The European Association for Cardio‐Thoracic Surgery supported this work.
Antonides, C. F. J. , Schoenrath, F. , de By, T. M. M. H. , Muslem, R. , Veen, K. , Yalcin, Y. C. , Netuka, I. , Gummert, J. , Potapov, E. V. , Meyns, B. , Özbaran, M. , Schibilsky, D. , Caliskan, K. , and the EUROMACS investigators (2020) Outcomes of patients after successful left ventricular assist device explantation: a EUROMACS study. ESC Heart Failure, 7: 1085–1094. 10.1002/ehf2.12629.
References
- 1. Kirklin JK, Pagani FD, Kormos RL, Stevenson LW, Blume ED, Myers SL, Miller MA, Baldwin JT, Young JB, Naftel DC. Eighth annual INTERMACS report: special focus on framing the impact of adverse events. The Journal of Heart and Lung Transplantation 2017; 36: 1080–1086. [DOI] [PubMed] [Google Scholar]
- 2. de By T, Mohacsi P, Gahl B, Zittermann A, Krabatsch T, Gustafsson F, Leprince P, Meyns B, Netuka I, Caliskan K, Castedo E, Musumeci F, Vincentelli A, Hetzer R, Gummert J, EUROMACS members . The European Registry for Patients with Mechanical Circulatory Support (EUROMACS) of the European Association for Cardio‐Thoracic Surgery (EACTS): second report. Eur J Cardiothorac Surg 2018; 53: 309–316. [DOI] [PubMed] [Google Scholar]
- 3. Goldstein DJ, Maybaum S, MacGillivray TE, Moore SA, Bogaev R, Farrar DJ, Frazier OH, HeartMate II Clinical Investigators . Young patients with nonischemic cardiomyopathy have higher likelihood of left ventricular recovery during left ventricular assist device support. J Card Fail 2012; 18: 392–395. [DOI] [PubMed] [Google Scholar]
- 4. Dandel M, Weng Y, Siniawski H, Potapov E, Lehmkuhl HB, Hetzer R. Long‐term results in patients with idiopathic dilated cardiomyopathy after weaning from left ventricular assist devices. Circulation 2005; 112: I37–I45. [DOI] [PubMed] [Google Scholar]
- 5. Birks EJ, Tansley PD, Hardy J, George RS, Bowles CT, Burke M, Banner NR, Khaghani A, Yacoub MH. Left ventricular assist device and drug therapy for the reversal of heart failure. New Engl J Med 2006; 355: 1873–1884. [DOI] [PubMed] [Google Scholar]
- 6. George RS, Yacoub MH, Bowles CT, Hipkin M, Rogers P, Hallas C, Banner NR, Dreyfus G, Khaghani A, Birks EJ. Quality of life after removal of left ventricular assist device for myocardial recovery. The Journal of Heart and Lung Transplantation 2008; 27: 165–172. [DOI] [PubMed] [Google Scholar]
- 7. Frazier OH, Baldwin ACW, Demirozu ZT, Segura AM, Hernandez R, Taegtmeyer H, Mallidi H, Cohn WE. Ventricular reconditioning and pump explantation in patients supported by continuous‐flow left ventricular assist devices. The Journal of Heart and Lung Transplantation: The Official Publication of the International Society for Heart Transplantation 2015; 34: 766–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Knierim J, Heck R, Pieri M, Schoenrath F, Soltani S, Stawowy P, Dreysse S, Stein J, Müller M, Mulzer J, Dandel M, Falk V, Krabatsch T, Potapov E. Outcomes from a recovery protocol for patients with continuous‐flow left ventricular assist devices. J Heart Lung Transplant 2019; 38: 440–448. [DOI] [PubMed] [Google Scholar]
- 9. de By TM, Mohacsi P, Gummert J, Bushnaq H, Krabatsch T, Gustafsson F, Leprince P, Martinelli L, Meyns B, Morshuis M, Netuka I. The European Registry for Patients with Mechanical Circulatory Support (EUROMACS): first annual report. Eur J Cardiothorac Surg 2015; 47: 770–777 discussion 76‐7. [DOI] [PubMed] [Google Scholar]
- 10. Kormos RL, Cowger J, Pagani FD, Teuteberg JJ, Goldstein DJ, Jacobs JP, Higgins RS, Stevenson LW, Stehlik J, Atluri P, Grady KL, Kirklin JK. The Society of Thoracic Surgeons Intermacs database annual report: evolving indications, outcomes, and scientific partnerships. The Annals of Thoracic Surgery 2019; 107: 341–353. [DOI] [PubMed] [Google Scholar]
- 11. Wever‐Pinzon O, Drakos SG, McKellar SH, Horne BD, Caine WT, Kfoury AG, Li DY, Fang JC, Stehlik J, Selzman CH. Cardiac recovery during long‐term left ventricular assist device support. J Am Coll Cardiol 2016; 68: 1540–1553. [DOI] [PubMed] [Google Scholar]
- 12. Drakos SG, Mehra MR. Clinical myocardial recovery during long‐term mechanical support in advanced heart failure: insights into moving the field forward. The Journal of Heart and Lung Transplantation 2016; 35: 413–420. [DOI] [PubMed] [Google Scholar]
- 13. Birks EJ, George RS, Hedger M, Bahrami T, Wilton P, Bowles CT, Webb C, Bougard R, Amrani M, Yacoub MH, Dreyfus G, Khaghani A. Reversal of severe heart failure with a continuous‐flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation 2011; 123: 381–390. [DOI] [PubMed] [Google Scholar]
- 14. Mann DL, Barger PM, Burkhoff D. Myocardial recovery and the failing heart. J Am Coll Cardiol 2012; 60: 2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maybaum S, Mancini D, Xydas S, Starling RC, Aaronson K, Pagani FD, Miller LW, Margulies K, McRee S, Frazier OH, Torre‐Amione G, LVAD Working Group . Cardiac improvement during mechanical circulatory support: a prospective multicenter study of the LVAD Working Group. Circulation 2007; 115: 2497–2505. [DOI] [PubMed] [Google Scholar]
- 16. Drakos SG, Wever‐Pinzon O, Selzman CH, Gilbert EM, Alharethi R, Reid BB, Saidi A, Diakos NA, Stoker S, Davis ES, Movsesian M, Li DY, Stehlik J, Kfoury AG, UCAR (Utah Cardiac Recovery Program) Investigators . Magnitude and time course of changes induced by continuous‐flow left ventricular assist device unloading in chronic heart failure: insights into cardiac recovery. J Am Coll Cardiol 2013; 61: 1985–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Drakos SG, Kfoury AG, Selzman CH, Verma DR, Nanas JN, Li DY, Stehlik J. Left ventricular assist device unloading effects on myocardial structure and function: current status of the field and call for action. Curr Opin Cardiol 2011; 26: 245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Baba HA, Grabellus F, August C, Plenz G, Takeda A, Tjan TDT, Schmid C, Deng MC. Reversal of metallothionein expression is different throughout the human myocardium after prolonged left‐ventricular mechanical support. The Journal of Heart and Lung Transplantation 2000; 19: 668–674. [DOI] [PubMed] [Google Scholar]
- 19. Bruckner BA, Stetson SJ, Perez‐Verdia A, Youker KA, Radovancevic B, Connelly JH, Koerner MM, Entman ME, Frazier OH, Noon GP, Torre‐Amione G. Regression of fibrosis and hypertrophy in failing myocardium following mechanical circulatory support. The Journal of Heart and Lung Transplantation 2001; 20: 457–464. [DOI] [PubMed] [Google Scholar]
- 20. Blaxall BC, Tschannen‐Moran BM, Milano CA, Koch WJ. Differential gene expression and genomic patient stratification following left ventricular assist device support. J Am Coll Cardiol 2003; 41: 1096–1106. [DOI] [PubMed] [Google Scholar]
- 21. Rodrigue‐Way A, Burkhoff D, Geesaman BJ, Golden S, Xu J, Pollman MJ, Donoghue M, Jeyaseelan R, Houser S, Breitbart RE, Marks A, Acton S. Sarcomeric genes involved in reverse remodeling of the heart during left ventricular assist device support. The Journal of Heart and Lung Transplantation 2005; 24: 73–80. [DOI] [PubMed] [Google Scholar]
- 22. Ogletree‐Hughes ML, Stull LB, Sweet WE, Smedira NG, McCarthy PM, Moravec CS. Mechanical unloading restores β‐adrenergic responsiveness and reverses receptor downregulation in the failing human heart. Circulation 2001; 104: 881–886. [DOI] [PubMed] [Google Scholar]
- 23. Bruggink AH, van Oosterhout MFM, de Jonge N, Ivangh B, van Kuik J, Voorbij RHAM, Cleutjens JP, Gmelig‐Meyling FH, De Weger RA. Reverse remodeling of the myocardial extracellular matrix after prolonged left ventricular assist device support follows a biphasic pattern. The Journal of Heart and Lung Transplantation 2006; 25: 1091–1098. [DOI] [PubMed] [Google Scholar]
- 24. Li YY, Feng Y, McTiernan CF, Pei W, Moravec CS, Wang P, Rosenblum W, Kormos RL, Feldman AM. Downregulation of matrix metalloproteinases and reduction in collagen damage in the failing human heart after support with left ventricular assist devices. Circulation 2001; 104: 1147–1152. [DOI] [PubMed] [Google Scholar]
- 25. Thohan V, Stetson SJ, Nagueh SF, Rivas‐Gotz C, Koerner MM, Lafuente JA, Loebe M, Noon GP, Torre‐Amione G. Cellular and hemodynamics responses of failing myocardium to continuous flow mechanical circulatory support using the DeBakey–Noon left ventricular assist device: a comparative analysis with pulsatile‐type devices. The Journal of Heart and Lung Transplantation 2005; 24: 566–575. [DOI] [PubMed] [Google Scholar]
- 26. Dipla K, Mattiello Julian A, Jeevanandam V, Houser Steven R, Margulies KB. Myocyte recovery after mechanical circulatory support in humans with end‐stage heart failure. Circulation 1998; 97: 2316–2322. [DOI] [PubMed] [Google Scholar]
- 27. Noguchi T, Hünlich M, Camp Phillip C, Begin Kelly J, El‐Zaru M, Patten R, Leavitt BJ, Ittleman FP, Alpert NR, LeWinter MM, VanBuren P. Thin filament‐based modulation of contractile performance in human heart failure. Circulation 2004; 110: 982–987. [DOI] [PubMed] [Google Scholar]
- 28. Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: a review of clinical, cellular, and molecular effects. Circ Heart Fail 2011; 4: 224–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Dandel M, Weng Y, Siniawski H, Stepanenko A, Krabatsch T, Potapov E, Lehmkuhl HB, Knosalla C, Hetzer R. Heart failure reversal by ventricular unloading in patients with chronic cardiomyopathy: criteria for weaning from ventricular assist devices. Eur Heart J 2011; 32: 1148–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]


