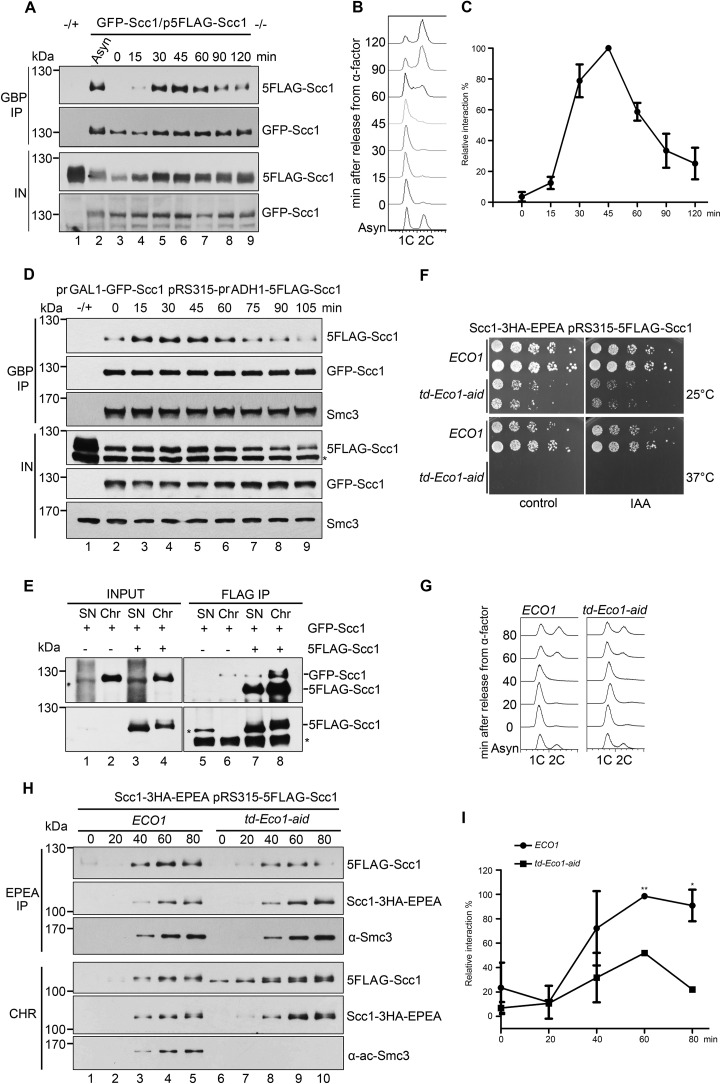
Figure 4.
Cohesin-cohesin interaction is regulated by Eco1 during the cell cycle. A, the GFP-SCC1/p5FLAG-SCC1 cells were grown, synchronized in G1 by α-factor (0 min), and released into S phase at 25 °C for the indicated time. The cell lysates were subjected to GBP-IP and IB against anti-FLAG and anti-GFP antibodies. B, a representative cell cycle profile analyzed by flow cytometry of the samples used in A. C, quantification of the relative intermolecular interaction of cohesin during the cell cycle. The densities of the FLAG-Scc1 and GFP-Scc1 bands in the precipitates were quantified. The ratio of FLAG-Scc1/GFP-Scc1 was calculated to indicate the relative cohesin-cohesin interaction in each sample. The maximum percentage among all samples was normalized to 100%. To ensure that the signals were within the linear range, immunoblots with appropriate exposure were quantified by Quantity One (Bio-Rad). Data shown are the mean ± S.D. (error bars) of three biological replicates. D, both GFP-SCC1 and 5FLAG-SCC1 under control of the GAL1 promoter were overexpressed in α-factor–arrested cells by galactose. All other experimental conditions were the same as described in A. E, the exponentially grown GFP-SCC1/p5FLAG-SCC1 cells were collected and fractionated into native Chr and nonchromatin-bound SN as described under “Experimental procedures.” Both SN and Chr fractions were subjected to FLAG-IP and IB using anti-Scc1 antibodies and anti-FLAG antibodies. 5FLAG-Scc1 cannot be well-detected in INPUT fraction using anti-Scc1 antibodies. Bottom, 5FLAG-Scc1 detected by anti-FLAG antibodies. F, efficient depletion of Eco1 via combined td and aid degrons leads to cell death. The growth of WT (ECO1) and Eco1 depletion strains (td-ECO1-aid) was examined by spotting on the medium with or without IAA at either 25 or 37 °C. G, Eco1 depletion causes only subtle changes in the cell-cycle progression. Shown are representative cell-cycle profiles of WT and Eco1 depletion strains used in H. After release from G1 arrest, cells were collected at the indicated time at 37 °C and analyzed by flow cytometry. H, Eco1 depletion interferes with cohesin-cohesin interaction on chromatin. Synchronized cells were prepared as in G. Native Chr was prepared as described under “Experimental procedures.” Scc1–3HA-EPEA was then precipitated via a C-tag affinity matrix and probed with the indicated antibodies. I, the relative cohesin-cohesin interaction in the presence or absence of Eco1 was quantified as described in C. Data shown are the mean ± S.D. of three biological replicates. *, p < 0.05; **, p < 0.01.