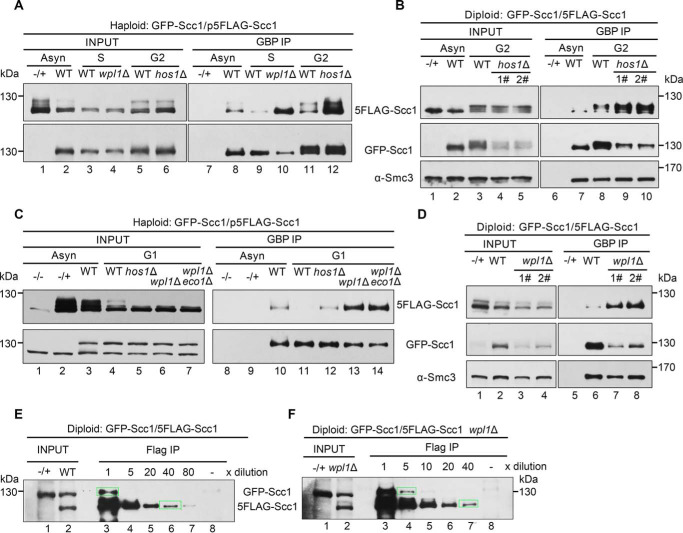
Figure 5.
The dimerized cohesin increases once wpl1 or hos1 is depleted. A, the haploid WT (GFP-Scc1/p5FLAG-Scc1), wpl1Δ or hos1Δ cells were grown with or without synchronization. The S phase cells were obtained by α-factor arrest and release for 60 min at 30 °C, whereas the G2 cells were arrested by nocodazole. GFP-Scc1 was precipitated via GBP beads from WCE. The proteins were detected via IBs against the indicated antibodies. −/+, control strain that does not harbor the GFP-tagged version of Scc1. B, the diploid WT (GFP-Scc1/5FLAG-Scc1) or hos1Δ cells were grown with or without G2 arrest. The lysates were subjected to GBP-IP and IB as above. 1# and 2# denote the biological repeats. C, the haploid WT (GFP-Scc1/p5FLAG-Scc1), hos1Δ, wpl1Δ, or wpl1Δeco1Δ cells were grown with or without G1 arrest. GBP-IPs and IBs were performed as above. D, the diploid WT (GFP-Scc1/5FLAG-Scc1) or wpl1Δ cells were grown exponentially. The lysates were subjected to GBP-IP and IB. 1# and 2# denote the biological repeats. E and F, the diploid WT (GFP-Scc1/5FLAG-Scc1) (E) or wpl1Δ (F) cells were grown exponentially. The lysates were precipitated by anti-FLAG M2 beads. A series of dilutions (5×, 10×, 20×, 40×, and 80×) of the samples were probed by anti-Scc1 antibodies. The indicated relative density of the band in a rectangular marquee was measured by Quantity One (Bio-Rad). The percentage of cohesin dimers was calculated as described under “Experimental procedures.” −/+, control strain that does not harbor the 5FLAG-tagged version of Scc1.