Figure 1.
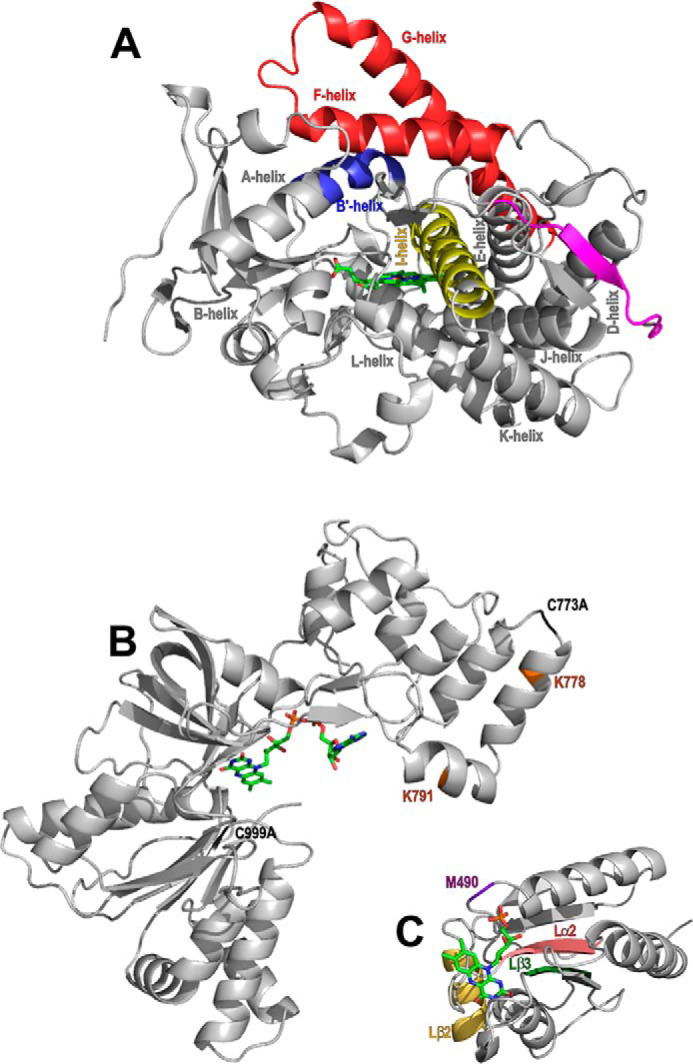
The structure and important features of P450 BM3 and its domains. The P450 BM3 heme domain is the catalytic domain and is presented as the 1BU7 structure (16). The CPR domain contains an FMN-binding domain and an FAD/NADPH-binding domain. The structures of these individual subdomains have been determined and are shown in the structures 1BVY (16) and 4DQK (15), respectively. A, the 55-kDa heme domain of P450 BM3 contains a heme prosthetic group (green) and is responsible for substrate binding and oxidative catalysis of the substrate. The I-helix (yellow) and the F/G-helices (red) are highly flexible components of the P450 fold. Other regions of the P450 fold shown are the mobile B′-helix (blue) and the heme domain C terminus (magenta) close to the heme prosthetic group (green). Other selected P450 α helices have also been labeled. B and C, the 65 kDa CPR domain obtains electrons from NADPH for the reductase activity of the enzyme. The CPR contains two subdomains; the FAD/NADPH-binding domain (B) and the FMN-binding domain (C). B, the two amino acid mutations required for inhibition of FAD domain dimer formation (C773A and C999A) that are necessary for successful FAD domain crystallization are highlighted in light green (15). In light blue, two lysine residues (Lys-778 and Lys-791) are colored brown and are found close to the CPR dimeric interface, as shown by EM experiments. The inter-CPR distances are ∼20 Å between Lys-778/Lys-791 in both cases (21). C, regions of the FMN-binding (flavodoxin) domain found to be important from dynamic simulations for electron transfer to the heme prosthetic group are highlighted in purple, wheat, dark green, and pink (32).
