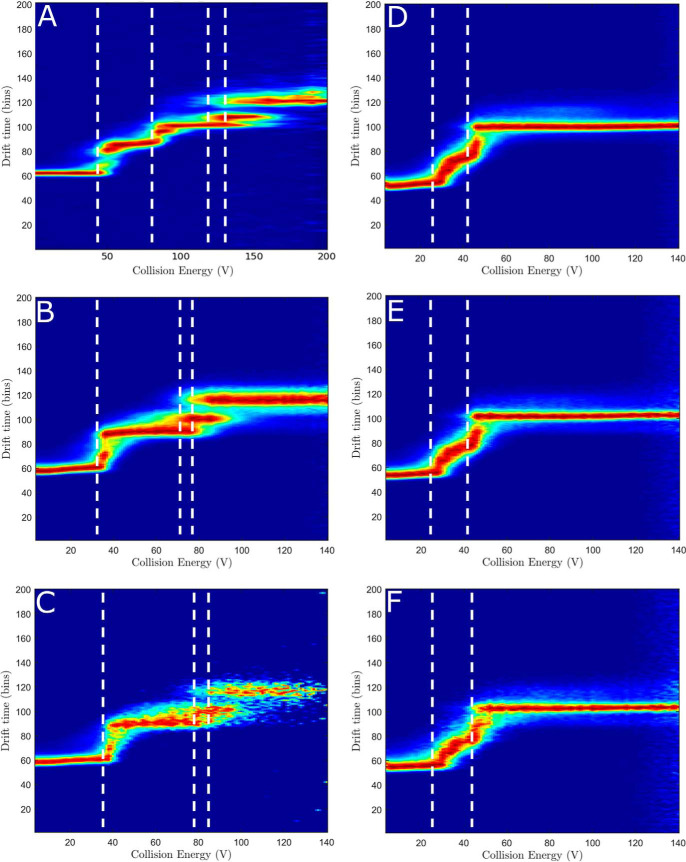
Figure 2.
Collision-induced unfolding of the full-length P450 BM3 enzyme and its P450 and reductase domains. CIU was undertaken on the full-length P450 BM3 using the most prevalent charge states found in the native MS studies, shown in Fig. S1. A, the ligand-free, full-length P450 BM3 using charge state [33+] shows six unfolding events. B, the ligand-free BM3 heme domain using charge state [14+] shows three unfolding events. C, the NPG-bound BM3 heme domain using charge state [14+] shows an increase in stability of the protein, as slightly more energy is required before unfolding is initiated. D, the BM3 reductase (CPR) domain using charge state [16+] shows a range of overlapping intermediates. E and F, the BM3 CPR domain bound to NADP+ using charge state [16+] shows no change to the unfolding pattern that was observed with the ligand-free CPR domain. The stoichiometry used was 1:1 (E) and 1:2 (F) protein to NADP+.