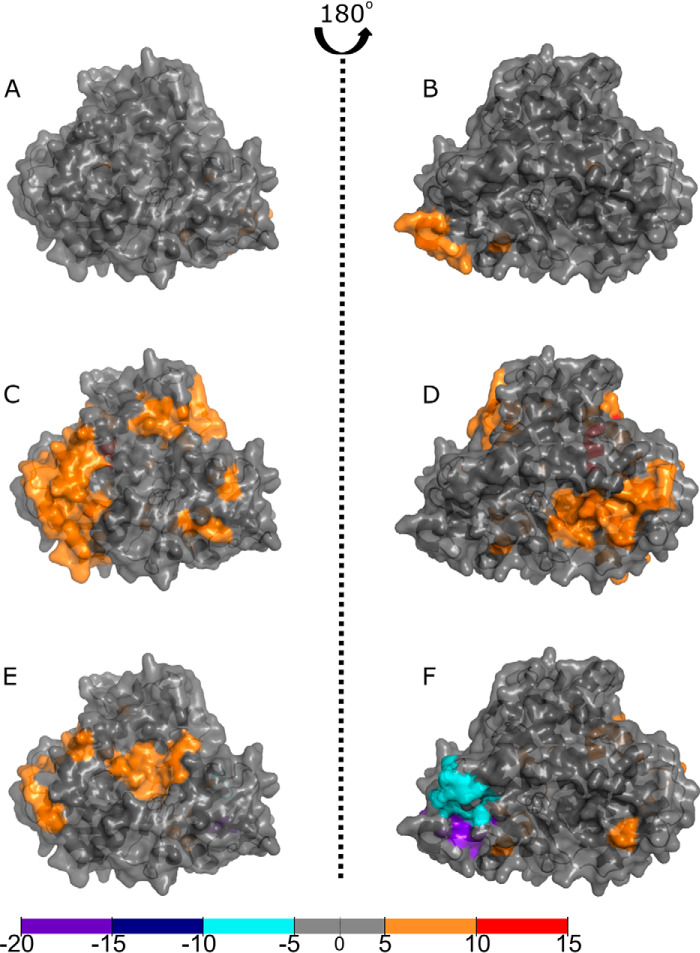
Figure 4.
Binding of ligands to full-length P450 BM3 elicits structural rearrangements across the heme domain as visualized by HDX-MS. Comparisons of the P450 BM3 heme domain with the full-length P450 BM3 enzyme in substrate-free or ligand-bound form reveal areas of increased shielding (cyan, dark blue, and purple) and solvent accessibility because of deshielding (orange and red). The crystal structure used is that of the NPG-bound heme domain (1JPZ). Residues with significant deuterium uptake changes are shown in Fig. S2. A and B, comparisons of the ligand-free heme domain with that of the NADP+-bound protein reveal very little change in the heme domain structure. C and D, comparisons of the ligand-free heme domain with the NPG-bound form of the protein show that the majority of the protein has undergone deshielding, as revealed by the widespread orange coloring. E and F, comparisons of the ligand-free heme domain to the NADP+/NPG-bound form of the protein show slight differences across the ligand-free heme domain compared with the NPG-bound form (C and D). Coverage maps for the HDX-MS heme domain data are shown in Fig. S4.