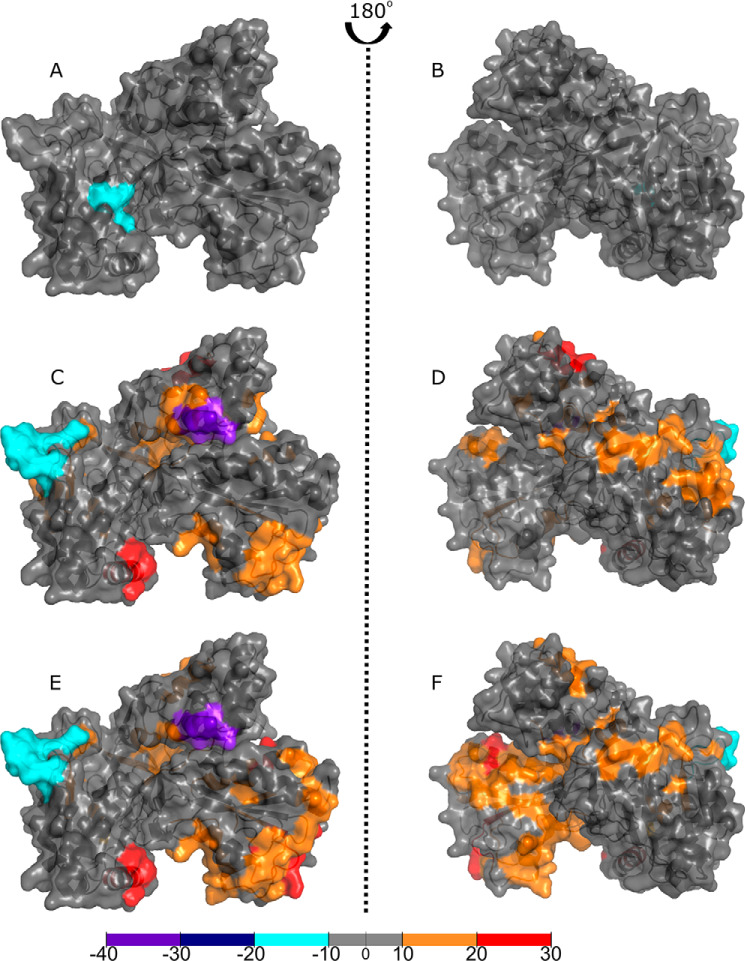
Figure 5.
Binding of ligands to full-length P450 BM3 elicits structural rearrangements across the CPR domain as visualized by HDX-MS. Comparisons of the CPR domain with the full-length P450 BM3 protein in the substrate-free or ligand-bound forms reveal areas of increased shielding (cyan, dark blue, and purple) and solvent accessibility because of deshielding (orange and red). The crystal structure used is the rat CPR model produced by aligning the BM3 FMN domain from the 1BVY structure and the BM3 FAD domain from the 4DQK structure to the rat CPR structure (1AMO). Residues with significant deuterium uptake changes are shown in Fig. S2. A and B, comparisons of the ligand-free CPR to the NADP+-bound form of the protein revealed little difference in solvent accessibility, except for one area of deshielding within the FAD domain. C and D, comparisons of the ligand-free CPR with the NPG-bound form of the full-length P450 BM3 protein showed that much of the CPR domain has undergone a change toward orange and red, indicating greater solvent accessibility that is likely because of the CPR domain adopting a less compact conformation. The greatest decrease in deuterium uptake is seen on the hinge domain of the FAD domain. In comparison, the greatest increase in deuterium uptake for the FAD-binding domain is located on the opposite face of the hinge domain (purple). E and F, binding of NPG elicits the greatest change across the CPR domain, whether NPG only or both NADP+/NPG were bound, as observed in C–F. There is a greater shift from gray to orange, indicating a larger conformational reorganization. The areas with the greatest shielding observed are present in the NPG- and NADP+/NPG-bound proteins. Coverage maps for the HDX-MS CPR domain data are shown in Figs. S3B, S5 and S6 describe, respectively, (i) P450 BM3 residues that exhibit highest degrees of change when full-length P450 BM3 is compared with its isolated heme and CPR domains; and (ii) P450 BM3 residues that exhibit highest degrees of change when comparing ligand-free and ligand-bound forms of P450 BM3.