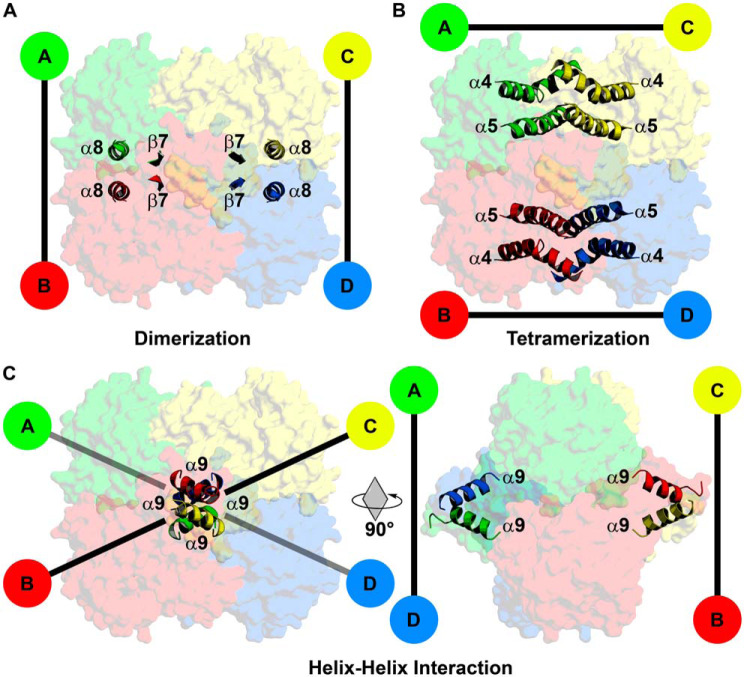
Figure 6.
Subunit interface domains of AfFabI. Specific structural domains link the protomers along the P, Q, and R axes interfaces. A, the dimerization domain consists of interacting α8 helices and β7 strands that link protomers A/B and C/D along the P axis. B, the tetramerization domain is an antiparallel four-helix bundle composed of α4 and α5 that links protomers A/C and B/D along the Q axis. C, the carboxyl-terminal α9 helix–helix interaction domain is a unique feature of AfFabI. This domain links protomers A/D and B/C along the R axis. Rotation of the tetramer by 90° around the Q axis enables clear visualization of the protomer–protomer interaction mediated by α9.