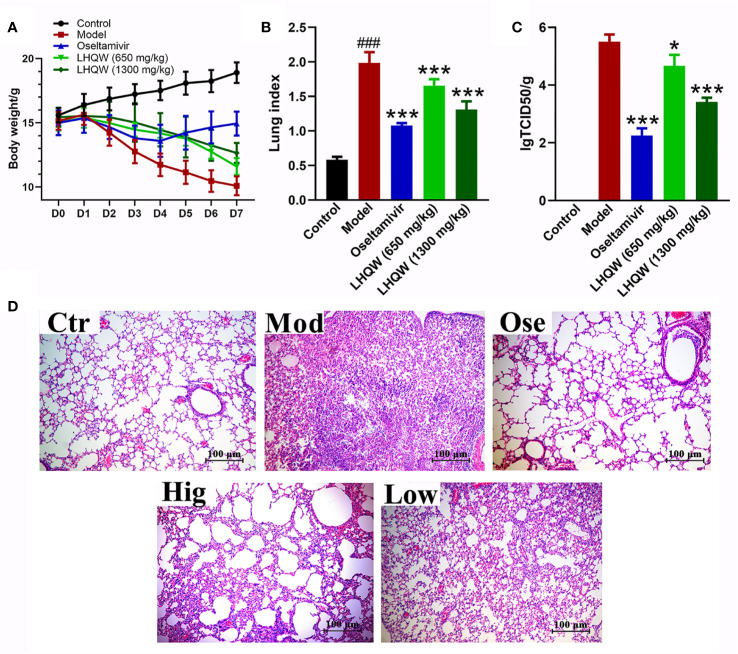
Figure 1.
LHQW alleviated H1N1-induced clinical signs in mice. (A) Bodyweight changes of mice infected by the HIN1 influenza virus. Mice were infected H1N1 (2 × LD50 per mouse) 2 h after the first administration and intragastrically administered twice per day (8-hour interval) for five consecutive days. The bodyweight of mouse was recorded daily and D0 weight was recorded before the day of first administration at the same time. (B) BALB/c mice were infected with the H1N1 virus (2×LD50 per mouse). At the fifth day of infection, seven mice were euthanized in each group. The mean lung index of each group was determined as lung weight/the final body weight. (C) Viral titers were determined by Reed–Muench method. Data are represented as the mean ± standard deviation (S.D.), n =7. ###p < 0.001 vs control group; *p < 0.05, ***p < 0.001 vs model group for one-way ANOVA. (D) Lung histology sections stained with hematoxylin and eosin (H&E stained, ×200 magnification) at the fifth-day post-infection. Ctr: the control group; Mod: the model group; Ose: the positive drug oseltamivir group; Hig: high dose of LHQW group (1,300 mg/kg/day); Low: low dose of LHQW group (650 mg/kg/day group).