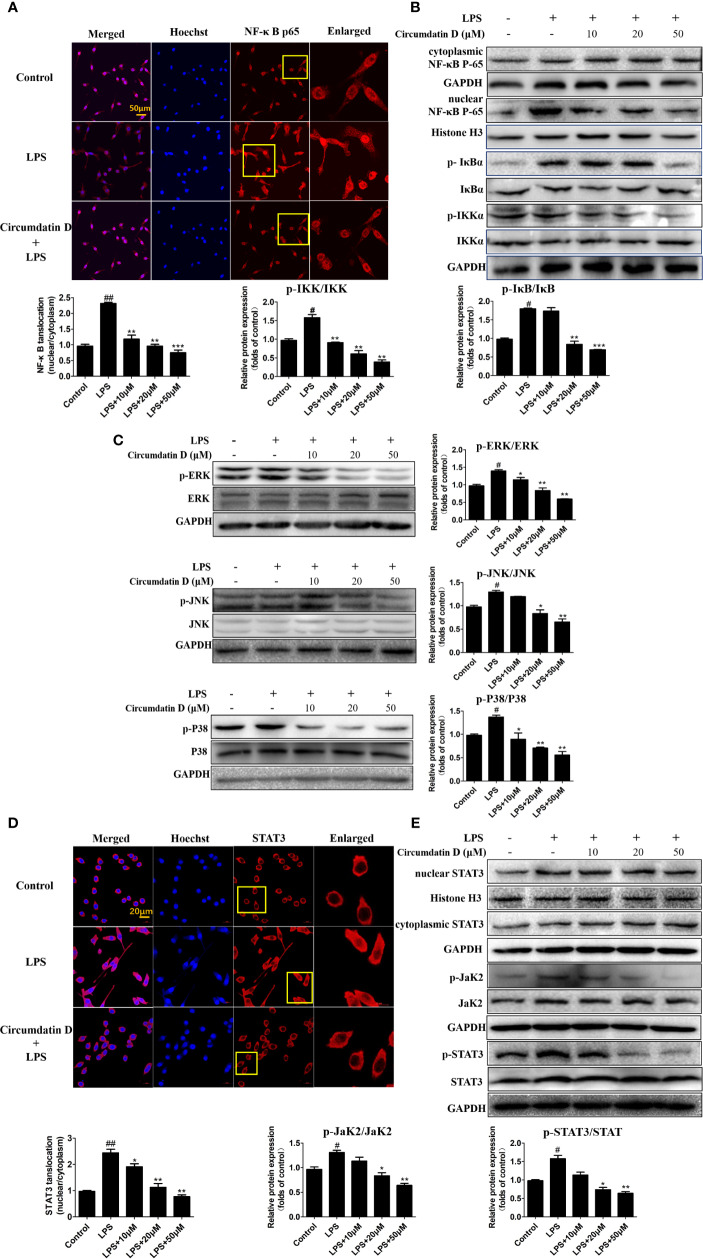
Figure 7.
Circumdatin D inhibition of NF-κB/STAT3 activation and MAPK phosphorylation in LPS-induced BV-2 cells. (A) BV-2 cells were stimulated with LPS (1 μg/ml) in the absence or presence of circumdatin D (10, 20 and 50 μM) for 3 h, followed by detection of the NF-κB p65 subunit translocation by immunocytochemistry. Red fluorescence represents the NF-κB p65 subunit, and blue fluorescence represents nuclear Hoechst staining. (B) BV-2 cells were stimulated with LPS (1 μg/ml) in the absence or presence of circumdatin D (10, 20 and 50 μM) for 3 h, NF-κB p65 levels in the nucleus and cytoplasm, the phosphorylated and total IKKβ and IκB proteins were determined by Western blot. Histone H3 and GAPDH were used as endogenous controls for nuclear and cytoplasmic proteins, respectively. (C) BV-2 cells were stimulated with LPS (1 μg/ml) in the absence or presence of circumdatin D (10, 20 and 50 μM) for 3 h, the phosphorylated and total ERK1/2-JNK1/2-p38 MAPKs were determined by Western blot. (D) BV-2 cells were treated with LPS (1 μg/ml) in the absence or presence of circumdatin D (10, 20 and 50 μM) for 3 h, followed by detection of the STAT3 translocation by immunocytochemistry. Red fluorescence represents the STAT3, and blue fluorescence represents nuclear Hoechst staining. (E) STAT3 levels in the nucleus and cytoplasm, the phosphorylated and total JaK2 and STAT3 proteins were determined by Western blot. Histone H3 and GAPDH were used as endogenous controls for nuclear and cytoplasmic proteins, respectively. Values represent the mean ± SD of three independent experiments (# compared with the control, *compared with LPS, */# P < 0.05, **/## P < 0.01, ***P < 0.001).