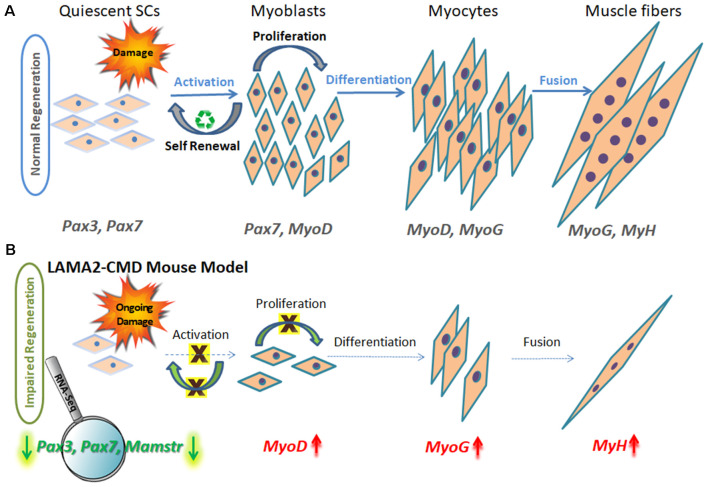
Figure 1.
Schematic model of muscle regeneration process in normal and LAMA2-CMD mouse model. (A) In healthy muscle, satellite cells (SCs) are in a quiescence state as a default, located in a specialized niche, between the sarcolemma and basal lamina. Quiescent SCs express Pax7, and a subpopulation expresses also Pax3 transcription factors; however, MyoD is not expressed at this stage. Upon injury, SCs are activated and undergo proliferation. SCs can perform asymmetric or symmetric divisions. Asymmetric division allows self-renewal and maintenance of SC pool, and symmetric division allows myogenesis and generating myoblasts. Following proliferation stage, myoblasts express MyoD transcription factor and exit the cell cycle to promote differentiation. Upon differentiation, myoblasts differentiate into elongated myocytes expressing myogenin (MyoG). Myocytes can fuse forming myotubes, which become myofibers, the contractile unit of muscle, and express developmental myosin-heavy chain marker (MyH). (B) In dystrophic muscle, SCs have limited ability to compensate for muscle damage. In LAMA2-CMD dy2J/dy2J mouse model, RNA-Seq data indicated little or even absent SC population, due to significant downregulation of Pax7 and Pax3 genes in quadriceps muscle of 8-week-old mice (Yanay et al., 2019). Absence of those transcription factors disable proper proliferation and self-renewal; thus, the entire regeneration process is impaired. MyoD and MyoG upregulation is postulated to be an attempt to compensate for the unbalanced process and the reduced repair of the damage.