Abstract
Objective
We aimed to determine if the dapivirine vaginal ring and the ring device alone (flexible silicone matrix polymer) was associated with the development of cervical cytology abnormalities.
Design
Secondary analysis comparing cervical cytology results between two randomized controlled microbicide trials (MTN-020/ASPIRE and MTN-003/VOICE).
Methods
Data from ASPIRE, a phase III, placebo-controlled trial of the dapivirine vaginal ring, were used in this analysis. Cervical cytology smears were evaluated at baseline and at the final visit with product use. We compared cytology results between women randomized to dapivirine versus placebo vaginal ring. We further assessed for the effect of the vaginal ring device on cervical cytology by comparing results with data from the oral placebo arm of VOICE, a prior HIV-1 prevention trial conducted in a similar population.
Results
Cervical cytology results for 2394 women from ASPIRE (1197 per study arm) were used in this analysis;median time between baseline and final visit with product use was 22.1 months. Cytology smear findings were comparable between dapivirine and placebo vaginal ring arms: at final visit, normal: 90.6 versus 91.5%, ASC-US//LSIL: 7.8 versus 7.4%, ASC-H/HSIL/AGC/AGC-favor neoplastic: 1.7 versus 1.1%, P = 0.44. Cytology data from VOICE had findings (normal: 87.8%, ASC-US/LSIL: 9.8%, ASC-H/HSIL/AGC/AGC-favor neoplastic: 2.4%) comparable with that of both dapivirine (P = 0.93) and placebo vaginal ring arms (P = 0.24).
Conclusion
These findings indicate that neither use of the dapivirine vaginal ring nor the vaginal ring device alone, over a period of 2 years, is associated with development of cervical cytology abnormalities that could lead to precancerous or cancerous lesions.
Keywords: cytology, dapivirine, preexposure prophylaxis, vaginal ring
Introduction
Antiretroviral medications used as preexposure prophylaxis (PrEP) can prevent acquisition of HIV type 1 (HIV-1) infection [1–3]. Longer acting methods of PrEP drug delivery, including vaginal rings, may improve adherence to PrEP and consequently provide greater HIV-1 protective effectiveness. A vaginal ring containing dapivirine, a nonnucleoside reverse-transcriptase inhibitor, was shown to provide HIV-1 protection in two phase III trials (MTN-020/ASPIRE and IPM-027/Ring Study), with HIV-1 incidence reduced by approximately 30% overall and by more than 50% for those who had objective evidence of ring use [4–6]. The effectiveness of the ring was further demonstrated in the subsequent open label extension studies (MTN-025/HOPE and IPM-032/DREAM), which suggested a 39–63% reduction in HIV-1 risk [7,8].
The dapivirine vaginal ring was shown to be well tolerated in both the phase III studies (systemic or reproductive system adverse events occurred with comparable frequency among those receiving the active ring versus placebo) and open label extension studies.
Qualitative data indicated that, despite initial fears about the ring’s appearance and potential side effects, study participants developed gradual familiarity with ring use through trial progression, and most reported that it was easy to use discretely and independently and to integrate into their lives [9]. The HOPE and DREAM studies also suggested participant interest in and adherence to the dapivirine vaginal ring when used in an open-label setting [7,8].
The dapivirine vaginal ring is a new innovation, and its uptake will depend on knowledge and acceptability in communities. Qualitative work exploring challenges with use of novel PrEP products has revealed multiple reasons for nonuse, with women’s concerns about safety being prominent. In qualitative analyses among women participating in ASPIRE, a common question raised by women was whether use of the ring could cause cervical cancer [10]. Cervical cancer is the most common cause of cancer among women in Africa where it accounts for 22% of all female cancers [11], and campaigns for cervical cancer prevention are common in many settings.
Cervical cancer is a multistep disease, the main cause of which is infection of the cervix with human papillomavirus (HPV). Contributing cofactors include multiple sexual partners, a compromised immune system and cervical inflammation [12]. Research with regard to the cervical cancer-causing potential of the dapivirine vaginal ring and vaginal rings in general is limited. Initial research with vaginally administered dapivirine (gel form) in rats over a period of 104 weeks indicated no neoplastic findings [13] and research with contraceptive vaginal rings indicated no significant changes in cervical cytology [14,15].
To better understand the effect of the dapivirine vaginal ring on cervical cytology abnormalities, we analyzed cervical cytology smear result data from the ASPIRE trial, comparing active dapivirine to placebo. In addition, to explore the effect of the vaginal ring device itself, we compared ASPIRE data to that from the MTN-003/VOICE trial, an earlier HIV-1 prevention study done in a similar population. We hypothesized that dapivirine and the vaginal ring alone would not cause cervical cytology abnormalities.
Methods
This is a secondary analysis of data from the ASPIRE trial (a randomized, double-blind, placebo-controlled phase III safety and effectiveness study of the dapivirine vaginal ring for HIV-1 prevention) and the VOICE trial (a randomized, double-blind, placebo-controlled phase IIB safety and effectiveness study of tenofovir 1% gel, tenofovir disoproxil fumarate tablet, and emtricitabine/tenofovir disoproxil fumarate tablet for HIV-1 prevention). Detailed methods and results for these trials have been previously published [4,16].
Briefly, between August 2012 and June 2014, 2629 healthy, sexually active, nonpregnant, HIV-1 uninfected women aged 18–45 years from Malawi, South Africa, Uganda, and Zimbabwe were enrolled in the ASPIRE trial. Eligible women were randomly assigned in a 1:1 ratio to receive either the dapivirine vaginal ring or placebo ring. For the VOICE trial, between September 2009 and June 2011, 5029 healthy, sexually active, nonpregnant, HIV-1 uninfected women aged 18–45 years from South Africa, Uganda, and Zimbabwe were enrolled. Eligible women were randomly assigned to one of five regimens – to remove any effect of medication or of use of an intravaginal product, only the oral placebo group (one-fifth of the trial population) was included in the present analysis. For both trials, participants provided written informed consent and applicable local and national ethical and regulatory authorities approved the study protocols.
In both the ASPIRE and VOICE studies, participants were assessed for cervical abnormalities using the cervical cytology smear test method and results were provided by local laboratories. Participants were not tested for HPV Cervical cytology testing was done at the trial screening visit (baseline) and at the final visit with product use. Results considered acceptable for study inclusion at screening were: negative, atypical squamous cells of undetermined significance (ASC-US); low-grade squamous intraepithelial lesion (LSIL), atypical squamous cells – cannot exclude high-grade squamous intraepithelial lesion (ASC-H) and atypical glandular cells (AGC). In both protocols, participants did not need to have a cytology smear during the screening period if they had a documented acceptable result within the 12 months prior to enrollment; thus, participants without a baseline cytology smear test result were assumed for the purposes of this analysis to have a normal result.
In order to determine if dapivirine or the vaginal ring alone, as a device, was associated with the development of cervical abnormalities, we evaluated cervical cytology smear results among three treatment arms across the two studies: the ASPIRE dapivirine vaginal ring arm; the ASPIRE placebo arm; and the VOICE oral placebo arm. We compared cervical cytology smear results between women randomized to dapivirine vaginal ring versus placebo vaginal ring at baseline and final visit with product use, to assess the potential effect of dapivirine exposure on cervical cytology smear results. We also compared baseline and final visit cervical cytology smear results within each vaginal ring arm to assess for change after vaginal ring use. Additional analyses included between arm comparisons of final visit cervical cytology smear results for those participants with a baseline LSIL result and baseline cytology smear results for those participants with a high-grade squamous intraepithelial lesion (HSIL) result. Data (for both vaginal ring arms) were then compared with that from the oral placebo arm of VOICE to assess for potential association between use of the vaginal ring device and cervical cytology abnormalities. As the goal of the treatment arm comparisons was to compare cervical cytology smear results after use of the respective treatment, we only considered from each arm the participants who had cervical cytology smear tests performed on or before the final visit with product use. HIV seroconverted participants were included in the analysis even though HIV-infection is known to increase the risk of abnormal cervical cytology and cervical cancer [17] to allow for inclusion of the maximum number of participants who were exposed to the study products and had cervical cytology smear results.
The primary outcome for these analyses was the result of the cervical cytology smear test, which was classified into eight categories, per the Bethesda system [18]: negative for intraepithelial lesion or cancer (malignancy); ASC-US; LSIL; ASC-H; HSIL; AGC; AGC – favor neoplastic; and carcinoma/cancer. There were no occurrences of cervical cytology smear results in the highest two categories, and because of the sparsity of results in some of the other categories, the categories were combined to create two practical outcome variables. The first outcome variable was a three-level variable: negative for intraepithelial lesion or cancer (malignancy); ASC-US/LSIL; and ASC-H/HSIL/AGC. These categories were chosen based on a seemingly natural breakpoint between low-grade and high-grade dysplasia, below which the presence of mildly abnormal cells tends to resolve spontaneously, and above which there may be a greater chance that a lesion may develop into cancer. The second outcome variable is a two-level variable considering any abnormal cervical cytology smear result (ASC-US or higher) versus a negative result.
Whenever appropriate, tests of marginal homogeneity and McNemar’s tests were used to assess individual participant changes in cervical cytology smear results from baseline to final visit. Baseline and some follow-up risk factors for abnormal cervical cytology smear results are presented overall and by the three-level cervical cytology smear result variable, with differences in the levels of the characteristic within each cervical cytology smear result category assessed by the chi-squared test for categorical variables and from the Kruskal–Wallis test for medians for continuous variables. For the comparison of the three-level cervical cytology smear result variable at baseline and last visit across the trial arms, a generalized linear model with multinomial distribution and a cumulative logit link was used. Unadjusted models were completed for ASPIRE, comparing the two arms; for comparisons between each ASPIRE vaginal ring arm and the oral placebo arm of VOICE, models with baseline outcomes were adjusted for baseline factors of age, country, education, ethnic group, self-employment, abnormal pelvic exam findings, cervical ectopy, use of injectable contraception, number of sexual partners, and the use of water in the practice of vaginal cleansing. Models for comparing cervical cytology smear results at the final visit were adjusted for baseline cervical cytology smear results and time from randomization to the final visit with cervical cytology smear results. Additional adjusted models were fit that included the same covariates as the baseline adjusted models as well as follow-up occurrences of Trichomonas vaginalis infection, chlamydial infection, HIV infection, and the cervical ectopy assessment, which occurred closest to the last visit with product use. These factors were selected based upon their availability in both studies, their possible association with the cervical cytology smear result outcome and based on a lack of collinearity with other covariates. Cochran–Mantel–Haenszel tests for general association were used for the between-arm comparisons of final visit cervical cytology smear results for those participants with a baseline LSIL result and baseline cytology smear results for those participants with a follow-up HSIL result. All statistical analyses were performed using SAS 9.4 (SAS Institute Inc., Cary, North Carolina, USA).
Results
Study sample
Baseline characteristics of the study participants are described in Table 1. In the ASPIRE placebo and dapivirine vaginal ring arms, the participant populations were similar with a median age of 26 years. The majority of participants had secondary education or more and almost half were earning their own income. More than half were unmarried, had had multiple pregnancies, were using injectable contraception or were conducting vaginal practices (any washing with soap or water). In the VOICE oral placebo arm, participants had a median age of 24 years. The majority had secondary education or more and more than half were earning their own income, unmarried, using injectable contraception or conducting vaginal practices. Almost half had had multiple pregnancies.
Table 1.
Baseline and follow-up characteristics of ASPIRE dapivirine and placebo arm participants and VOICE oral placebo arm participants.
| Participant characteristics | ASPIRE dapivirine vaginal ring arm (n = 1197) | ASPIRE placebo vaginal ring arm (n = 1197) | VOICE oral placebo arm (n = 673) |
|---|---|---|---|
| Baseline | |||
| Median age (years) (IQR) | 26 (22–31) | 26 (23–31) | 24 (21–29) |
| Ethnic group | |||
| Zulu | 467 (39%) | 455 (38%) | 370 (55%) |
| Shona | 290 (24%) | 282 (24%) | 103 (15%) |
| Xhosa | 104 (9%) | 114 (10%) | 51 (8%) |
| Other | 336 (28%) | 346 (29%) | 149 (22%) |
| Country | |||
| Malawi | 128 (11%) | 133 (11%) | NA |
| South Africa | 647 (54%) | 645 (54%) | 507 (75%) |
| Uganda | 116(10%) | 114 (10%) | 48 (7%) |
| Zimbabwe | 306 (26%) | 305 (25%) | 120 (18%) |
| Education | |||
| Primary or less | 200 (17%) | 186 (16%) | 66 (10%) |
| Secondary or more | 997 (83%) | 1011 (84%) | 607 (90%) |
| Earns own income | 558 (47%) | 531 (44%) | 408 (61%) |
| Self-employed | 256 (21%) | 251 (21%) | 78 (12%) |
| Married | 480 (40%) | 507 (42%) | 175 (26%) |
| Has had multiple pregnancies | 707 (59%) | 746 (62%) | 318 (47%) |
| Median number of male sexual partners, past 3 months (IQR) | 1 (1–1) | 1 (1–1) | 1 (1–1) |
| Current use of contraception | |||
| Hormonal | |||
| Injectable | 648 (54%) | 686 (57%) | 473 (70%) |
| Oral pills | 132 (11%) | 122 (10%) | 140 (21%) |
| Other | 422 (35%) | 391 (33%) | 63 (9%) |
| Vaginal practices, past 3 months | |||
| Any washing with soap | 238 (20%) | 282 (24%) | 238 (36%) |
| Any washing with water | 528 (44%) | 538 (46%) | 391 (59%) |
| Abnormalities on pelvic exam | 80 (7%) | 96 (8%) | 130 (19%) |
| Cervical ectopy ≥1% | 280 (23%) | 297 (25%) | 139 (21%) |
| Gonorrhea | 53 (4%) | 43 (4%) | 21 (3%) |
| Chlamydia | 155 (13%) | 124 (10%) | 83 (12%) |
| Trichomonas | 77 (6%) | 87 (7%) | 46 (7%) |
| Last follow-up visit | |||
| Cervical ectopy ≥1% | 220 (18%) | 219 (18%) | 36 (5%) |
| Gonorrhea | 133 (11%) | 144 (12%) | 24 (4%) |
| Chlamydia | 254 (21%) | 256 (21%) | 103 (15%) |
| Trichomonas | 137 (11%) | 149 (12%) | 63 (9%) |
| HIV-positive | 63 (5%) | 86 (7%) | 40 (6%) |
Cervical cytology smear collection was acceptable in the ASPIRE trial and follow-up results were available for 1197 participants from the dapivirine vaginal ring arm (91.2% of 1313 randomized) and 1197 from the placebo vaginal ring arm (91.0% of 1316 randomized). For the VOICE oral placebo arm, follow-up results were available for 673 (66.7%) of 1009 randomized. The median follow-up time to last cervical cytology smear result in ASPIRE was 672 days [interquartile range (IQR) = (426 837)] for the dapivirine vaginal ring group and 672 days [IQR = (448 836)] for the placebo vaginal ring group In the VOICE study, the median follow-up time to last cervical cytology smear result was 418 days [IQR = (343 519)].
Comparison between the dapivirine and placebo vaginal ring groups in ASPIRE
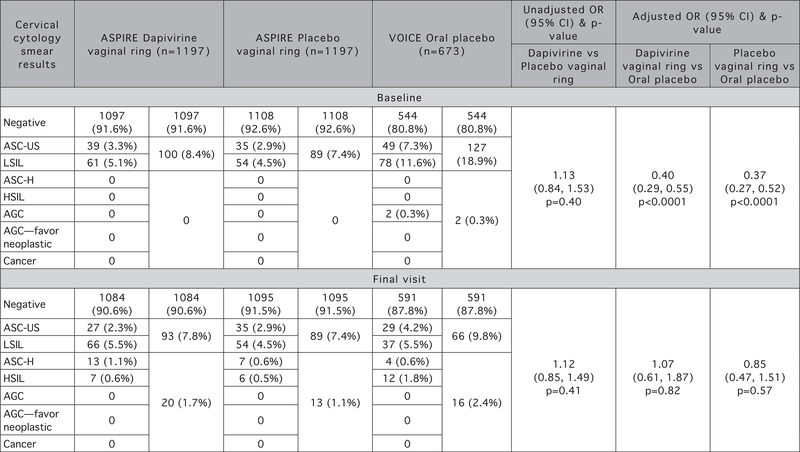
At baseline and at the final visit with product use, there were no statistically significant differences between cervical cytology smear result findings for the ASPIRE dapivirine and placebo vaginal ring arms (Fig. 1). At baseline, cervical cytology smear result findings were comparable between arms with 8.4% of dapivirine vaginal ring participants having ASC-US/LSIL findings compared with 7.4% among placebo vaginal ring participants (none had ASC-H/HSIL/AGC/AGC-favor neoplastic), [unadjusted odds ratio (OR) = 1.13, 95% confidence interval (CI) 0.84–1.53, P = 0.40]. At the final visit, cervical cytology smear result findings were similar, with a small number shifting towards higher grade findings (dapivirine vaginal ring participants having 7.8% ASC-US/LSIL and 1.7% ASC-H/HSIL/AGC/AGC-favor neoplastic findings compared with 7.4 and 1.1%, respectively among placebo vaginal ring participants, unadjusted OR = 1.12, 95% CI 0.85–1.49, P = 0.41). In addition, no significant differences were observed between the proportion of participants with baseline abnormal cervical cytology smear results and last visit abnormal cervical cytology smear results in both vaginal ring arms: dapivirine vaginal ring: 8.4% baseline versus 9.5% last visit, McNemar’s test, P = 0.31 and placebo vaginal ring arm: 7.4% baseline versus 8.5% last visit, McNemar’s test, P = 0.30.
Fig. 1.
Comparison of cervical cytology smear results between ASPIRE vaginal ring arms and between each vaginal ring arm and the VOICE oral placebo arm at baseline and final visit. P-value for comparison of the three-level cervical cytology smear result variable at baseline and final visit between dapivirine vaginal ring and placebo vaginal ring, between placebo vaginal ring and VOICE oral placebo and between dapivirine vaginal ring and VOICE oral placebo from generalized linear model with multinomial distribution and a cumulative logit link. AGC, atypical glandular cells; ASC-H, atypical squamous cells - cannot exclude high-grade squamous intraepithelial lesion; ASC-US, atypical squamous cells of undetermined significance; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Comparison of final visit cervical cytology smear results for those participants with a baseline LSIL result showed no significant difference (P = 0.90) with majority reverting to a negative result (70% in the dapivirine arm and 78% in the placebo arm) (Table 2). Comparison of baseline cytology smear results for those participants with a final visit HSIL result was similarly not significant (P = 0.89) with majority of HSIL arising from participants with a negative result at baseline (57% in the dapivirine arm and 67% in the placebo arm) (Table 3).
Table 2.
Comparison of final visit cytology smear results for ASPIRE dapivirine vaginal ring arm, placebo vaginal ring arm and VOICE oral placebo arm participants with a baseline low-grade squamous intraepithelial lesion result.
| LSIL at baseline |
P-value |
|||||
|---|---|---|---|---|---|---|
| Final visit cytology smear results | Dapivirine vaginal ring (n = 61) | Placebo vaginal ring (n = 54) | Oral placebo (n = 78) | Dapivirine versus placebo vaginal ring | Dapivirine vaginal ring versus oral placebo | Placebo vaginal ring versus oral placebo |
| Negative for intraepithelial lesion | 43 (70%) | 42 (78%) | 55 (71%) | 0.90 | 0.66 | 0.65 |
| ASC-US | 2 (3%) | 2 (4%) | 4 (5%) | |||
| LSIL | 12 (20%) | 8 (15%) | 12 (15%) | |||
| ASC-H | 2 (3%) | 1 (2%) | 1 (1%) | |||
| HSIL | 2 (3%) | 1 (2%) | 1 (8%) | |||
P-value for comparison of final visit cytology smear results for ASPIRE dapivirine vaginal ring arm, placebo vaginal ring arm and VOICE oral placebo arm participants with a baseline LSIL result is from Cochran–Mantel–Haenszel tests for general association. ASC-H, atypical squamous cells – cannot exclude high-grade squamous intraepithelial lesion; ASC-US, atypical squamous cells of undetermined significance; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Table 3.
Comparison of baseline cytology smear results for ASPIRE dapivirine vaginal ring arm, placebo vaginal ring arm and VOICE oral placebo arm participants with a final visit high-grade squamous intraepithelial lesion result.
| HSIL at final visit |
P-value |
|||||
|---|---|---|---|---|---|---|
| Baseline cytology smear results | Dapivirine vaginal ring (n = 7) | Placebo vaginal ring (n = 6) | Oral placebo (n = 12) | Dapivirine versus placebo vaginal ring | Dapivirine vaginal ring versus oral placebo | Placebo vaginal ring versus oral placebo |
| Negative for intraepithelial lesion | 4 (57%) | 4 (67%) | 5 (42%) | 0.89 | 0.67 | 0.41 |
| ASC-US | 1 (14%) | 1 (17%) | 1 (8%) | |||
| LSIL | 2 (29%) | 1 (17%) | 6 (50%) | |||
P-value for comparison of baseline cytology smear results for ASPIRE dapivirine vaginal ring arm, placebo vaginal ring arm and VOICE oral placebo arm participants with a final visit HSIL result is from Cochran–Mantel–Haenszel tests for general association. ASC-US, atypical squamous cells of undetermined significance; HSIL, high-grade squamous intraepithelial lesion; LSIL, low-grade squamous intraepithelial lesion.
Comparison of vaginal ring groups in ASPIRE with the oral placebo group in VOICE
For VOICE oral placebo arm participants, unlike in ASPIRE, the presence of cervical abnormalities decreased from the baseline to the final visit overall, but, like ASPIRE, there was a small number who had higher grade dysplasia at the final visit. Specifically, the distribution of cervical cytology smear results was significantly different at the final visit than at baseline with a significantly higher proportion of participants exhibiting abnormal cervical cytology smear results at baseline (19.2% baseline versus 12.2% final visit, McNemar’s test, P = 0.0001). However, there was also a significantly higher proportion of participants who had an ASC-H, HSIL or AGC at final visit (2.4%) than at baseline (0.3%, McNemar’s test, P = 0.001) (Fig. 1).
When comparing final visit cervical cytology smear results between ASPIRE dapivirine vaginal ring participants and VOICE oral placebo arm participants, there was no evidence of a difference in the rate of abnormal cervical cytology smear results between study arms in a model adjusted only by baseline cervical cytology smear results and time to last visit cervical cytology smear results (OR = 0.98, 95% CI 0.69–1.38, P = 0.89). Furthermore, no evidence of a difference was detected after adjustment for all potential baseline and follow-up confounders (adjusted OR = 1.07, 95% CI 0.61–1.87, P = 0.82). Similarly, when comparing final visit cervical cytology smear results between ASPIRE placebo vaginal ring participants and VOICE oral placebo arm participants, there was no evidence of a difference between study arms in a model adjusted only by baseline cervical cytology smear results and time to last visit cervical cytology smear results (OR = 0.91, 95% CI 0.63–1.30, P = 0.59), nor after adjustment by all potential baseline and follow-up confounders (adjusted OR = 0.85, 95% CI 0.47–1.51, P = 0.57) (Fig. 1).
Comparison of final visit cervical cytology smear results for dapivirine vaginal ring arm participants with a baseline LSIL result with those in the VOICE oral placebo arm showed no significant difference (P = 0.66) with the majority reverting to a negative result. A similar observation was made when comparing the placebo vaginal ring arm and oral placebo arm (P = 0.65) (Table 2). Comparison of baseline cytology smear results for those participants with a HSIL result was similarly not significant (dapivirine vaginal ring arm versus oral placebo arm: P = 0.67; placebo vaginal ring arm versus oral placebo arm: P = 0.41); however, there appeared to be a higher number of HSIL results at final visit arising from participants in the VOICE oral placebo with a LSIL result at baseline (50%) (Table 3).
Overall, there was no evidence of an association between use of the dapivirine and placebo vaginal ring and abnormal cervical cytology smear results in ASPIRE when compared with the oral placebo arm in VOICE.
Discussion
In this analysis of data from two large clinical trials, we found no evidence that use of the dapivirine vaginal ring results in cervical cytology abnormalities. Our approach was rigorous in its use of placebo-controlled clinical trial data, and we were able to leverage data from a second clinical trial conducted in a similar population to assess any potential contribution of the ring device itself.
Cervical cancer, and its premalignant precursor cervical intraepithelial neoplasia are known to be caused by the persistence of strains of HPV and factors that correlate with higher persistence rates include age, immunodeficiency, smoking, long-term oral contraceptive use and Chlamydia trachomatis infection as well as cervicovaginal microbiota [19].
The higher proportion of participants with abnormal cervical cytology smear results at baseline observed in the VOICE oral placebo arm is mostly attributable to transient HPV infections reported as ASC-US/LSIL, which is very common among adolescents and young women. The decrease in abnormalities observed at the final visit is likely because of clearance of the HPV infection and associated ASC-US/LSIL, which is often rapid, with more than half of infections clearing within a year, and 90% of infections within approximately 2 years of acquisition [20]. The higher proportion of participants with cervical abnormalities at final visit in the VOICE group could possibly be due to the shorter time of follow-up between baseline and final visit in VOICE (±14 months) compared with ASPIRE (±22.4 months). It is not clear if the advancement of the severity of abnormalities over time observed among a small proportion of participants (<2%) are clinically relevant, or perhaps expected in the natural course of abnormal cell changes toward cancer.
Limitations of this analysis include that it is a comparison of data across two studies (with multiple sites) having a discrete follow-up period, slightly different populations, variability of conducting follow-up procedures, and collection of slightly different data elements regarding risk factors for abnormal cervical cytology smear results (e.g. the VOICE study did not capture information about exposure to smoking, another risk factor for cell changes in the cervix). In addition, standardized training was not conducted across all participating sites and each study site utilized local thresholds for cytology categories. As there was no dedicated cervical cytology smear result data collection item for baseline results in ASPIRE (as was done in VOICE), baseline cervical cytology smear data for these analyses were captured from the preexisting conditions case report form where only abnormal results are recorded and lack of recorded information assumed normal results. These study-defined data collection methods may have resulted in an underestimation of the number of abnormal baseline cervical cytology smear results in ASPIRE when compared with VOICE. In addition, participants in both the VOICE and ASPIRE studies were not tested for HPV, a known risk factor for having an abnormal cervical cytology smear result [19]. Despite these limitations, the randomized comparison within ASPIRE, and the near-complete data collection at the final visit, after product exposure, provide high rigor for this analysis.
In conclusion, these findings indicate that neither use of the dapivirine vaginal ring nor the vaginal ring device itself, over a period of 2 years, is associated with development of cervical cytology abnormalities that could lead to precancerous or cancerous lesions. These data contribute positively to the growing body of knowledge regarding safety of the dapivirine vaginal ring and will assist in addressing issues of vaginal ring nonadherence related to cervical cancer development among participants in other related trials as well as in educating communities regarding this promising HIV prevention product.
Acknowledgements
The authors are grateful to the study participants for their participation and dedication. The authors thank the research site study team members, the MTN-020/ASPIRE and MTN-003/VOICE Protocol Management teams, the MTN Leadership Operations Center and the Statistical Center for HIV/AIDS Research and Prevention for their contributions to data collection.
The MTN-020/ASPIRE and MTN-003/VOICE trials were designed and implemented by the Microbicide Trials Network (MTN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, and UM1AI106707), with cofunding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the US National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The vaginal rings used in the ASPIRE study were developed and supplied by the International Partnership for Microbicides (IPM). The oral placebo used in the VOICE study were supplied by Gilead Sciences.
Funding: Division of AIDS, US National Institute of Allergy and Infectious Diseases; US National Institute of Child Health and Human Development; US National Institute of Mental Health; US National Institutes of Health.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Thigpen MC, Kebaabetswe PM, Paxton LA, Smith DK, Rose CE, Segolodi TM, et al. , TDF2 Study Group. Antiretroviral preexposure prophylaxis for heterosexual HIV transmission in Botswana. N Engl J Med 2012;367:423–434. [DOI] [PubMed] [Google Scholar]
- 2.Abdool Karim Q, Abdool Karim SS, Frohlich JAS, Grobler AC, Baxter C, Mansoor LE, et al. , CAPRlSA 004 Trial Group. Effectiveness and safety of tenofovir gel, an antiretroviral microbicide, for the prevention of HIV infection in women. Science 2010;329:1168–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grant RM, Lama JR, Anderson PL, McMahan V, Liu AY, Vargas L, et al. , iPrEx Study Team. Preexposure chemoprophylaxis for HIV prevention in men who have sex with men. N Engl J Med 2010;363:2587–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baeten JM, Palanee-Phillips T, Brown ER, Schwartz K, Soto-Torres LE, Govender V, et al. , MTN-020-ASPIRE Study Team. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med 2016;375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nel A, van Niekerk N, Kapiga S, Bekker LG, Gama C, Gill K, et al. , Ring Study Team. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med 2016; 375:2133–2143. [DOI] [PubMed] [Google Scholar]
- 6.Brown E Residual dapivirine ring levels indicate higher adherence to vaginal ring is associated with HIV-1 protection. 21st International AIDS Conference 2016: Durban, South Africa. [Google Scholar]
- 7.Baeten J High adherence and sustained impact on HIV-1 incidence: Final results of an open-label extension trial of the dapivirine vaginal ring. 10th International AIDS Society Conference on HIV Science (IAS 2019) 2019. Mexico City, Mexico. [Google Scholar]
- 8.Nel A Safety, adherence and HIV-1 seroconversion in DREAM - an open-label dapivirine vaginal ring trial. 9th South African AIDS Conference, 2019, Durban, South Africa. [Google Scholar]
- 9.Montgomery ET, van der Straten A, Chitukuta MS, Reddy K, Woeber K, Atujuna M, et al. , MTN-020/ASPIRE Study. Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS 2017;31:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montgomery ET, Stadler J, Naidoo S, Katz AWK, Laborde N, Garcia M, et al. Reasons for nonadherence to the dapivirine vaginal ring: narrative explanations of objective drug-level results. AIDS 2018;32:1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Africa, World Health Organization. Cervical cancer common amongst African women. 2017. Available at: http://www.afro.who.int/news/cervical-cancer-common-amongst-african-women [Accessed: 11 March 2018]
- 12.Sales KJ, Katz AA. Inflammatory pathways in cervical cancer - the UCT contribution. S African Med J, [Sl] 2012;102:493–496. [DOI] [PubMed] [Google Scholar]
- 13.Dapivirine Vaginal Ring Investigator’s Brochure, I.P.f. Microbicides, 2018. [Google Scholar]
- 14.Wieder DR, Pattimakiel L. Examining the efficacy, safety, and patient acceptability of the combined contraceptive vaginal ring (NuvaRing). Int J Womens Health 2010;2:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser IS, Fraser IS, Baker J, Archer D, Landgren BM, Killick S, et al. Vaginal epithelial surface appearances in women using vaginal rings for contraception. Contraception 2000; 61:131–138. [DOI] [PubMed] [Google Scholar]
- 16.Marrazzo JM, Ramjee G, Richardson BA, Gomez K, Mgodi N, Nair G, et al. , VOICE Study Team. Tenofovir-based preexposure prophylaxis for HIV infection among African Women. New Engl J Med 2015;372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chirenje ZM. HIV and cancer of the cervix. Best Pract Res Clin Obstet Gynaecol 2005;19:269–276. [DOI] [PubMed] [Google Scholar]
- 18.Apgar BS, Zoschnick L, Wright TC Jr. The 2001 Bethesda System terminology. Am Fam Physician 1992-;68:8. [PubMed] [Google Scholar]
- 19.Mitra A, MacIntyre DA, Marchesi JR, Lee YS, Bennett PR, Kyrgiou M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: what do we know and where are we going next? Microbiome 2016;4:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schiffman M, Wentzensen N. Human papillomavirus infection and the multistage carcinogenesis of cervical cancer. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2013;22: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]