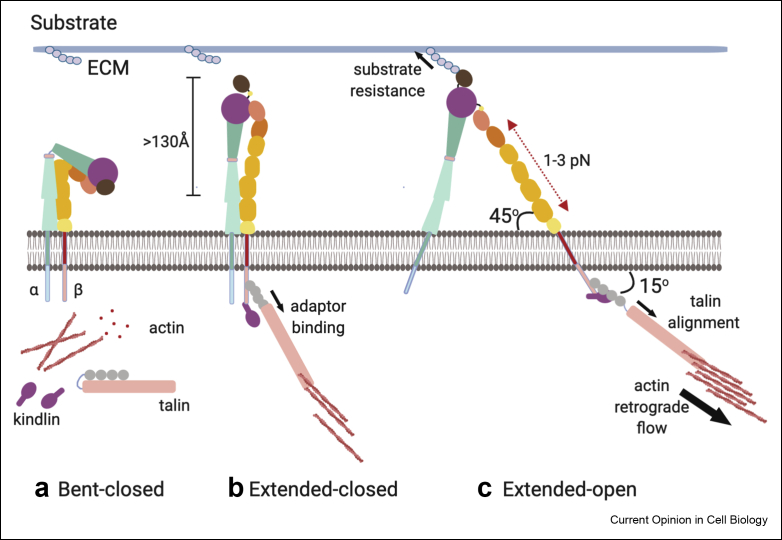
Figure 1.
New insights into integrin activation. Integrins exist in three states: (a) bent-closed, an inactive conformation where the integrin is not engaged with its ECM ligand; (b) ‘extended-closed’, a low affinity, intermediate state that may arise from talin and/or kindlin binding; (c) ‘extended-open’, elicited by simultaneous binding of ECM ligand and intracellular adaptors associated with the actin cytoskeleton. Intracellular adaptor binding leads to a >130 Å extension of integrin conformation [4,12]. Resistive forces from ligand binding and cytoskeletal adaptor interactions (thin black arrows) exert 1–3 pN tensile forces on the integrin (red double arrows). The direction of actin retrograde flow (thick black arrows) generates tension on talin positioning it 150 to the plasma membrane and drives the tilting of the integrin β subunit to an angle ∼450 to the plasma membrane aligning it with the F-actin filaments [19,30]. This extended, tilted integrin orientation establishes equilibria along its force-bearing axis and stabilises the high-affinity ligand binding state. Based predominantly on data taken from LFA-1 and ICAM-1 binding studies. ICAM-1, Intercellular Adhesion Molecule-1.