Figure 2.
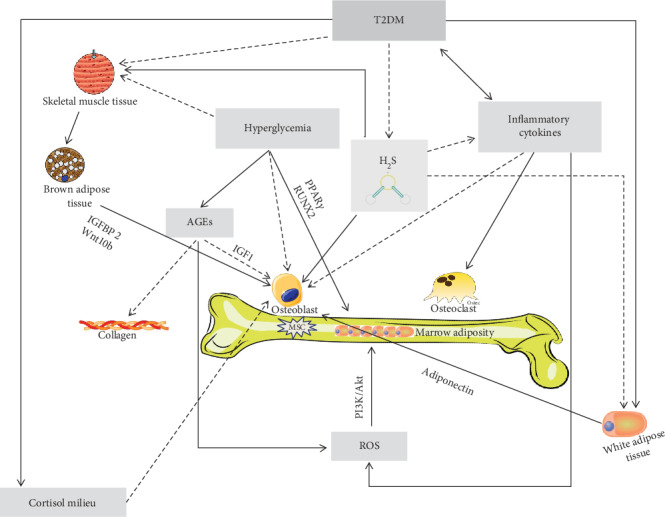
Mechanisms underlying bone fragility in type 2 diabetes mellitus (T2DM). In T2DM, the muscle tissue reduction, due to several factors including hyperglycemia per se, but probably also hydrogen sulfide (H2S) decrease, is thought to have a negative role on osteoblast lineage, via its crosstalk with the brown adipose tissue. Indeed, the muscle tissue is known to influence the brown adipose tissue, physiologically stimulating the secretion of factors (such as IGFBP2 and Wnt10b) thought to be important for osteoblast proliferation and activity. Osteoblast differentiation and activity, in T2DM, may be also impaired directly by the reduction of H2S levels that physiologically are thought to stimulate the osteoblast lineage. Hyperglycemia may directly reduce bone mesenchymal stem cell (MSC) viability and clonogenicity and also have an indirect negative effect on osteoblasts via the accumulation of advanced glycation end products (AGEs), which negatively affects osteoblasts through a reduction of the insulin-like growth factor-1 (IGF1) levels. The AGE accumulation impairs the normal collagen formation and leads to reactive oxygen species (ROS) increase that may augment marrow adiposity via the phosphoinositide-3-kinase–protein kinase B/Akt (PI3K/Akt) pathway. The inflammatory cytokine increase, directly and/or indirectly (due to the H2S reduction), may also impair osteoblastogenesis and increase osteoclast activity and ROS, ultimately leading to bone adiposity. Finally, in T2DM, osteoblasts may be also damaged by the low adiponectin levels due to the increase of white adipose tissue, which is a characteristic of T2DM itself but also a consequence of low H2S levels. Finally, even an altered cortisol secretion, peripheral activation, and sensitivity (i.e., “cortisol milieu”) have been suggested to potentially impair osteoblast activity.
