Figure 7:
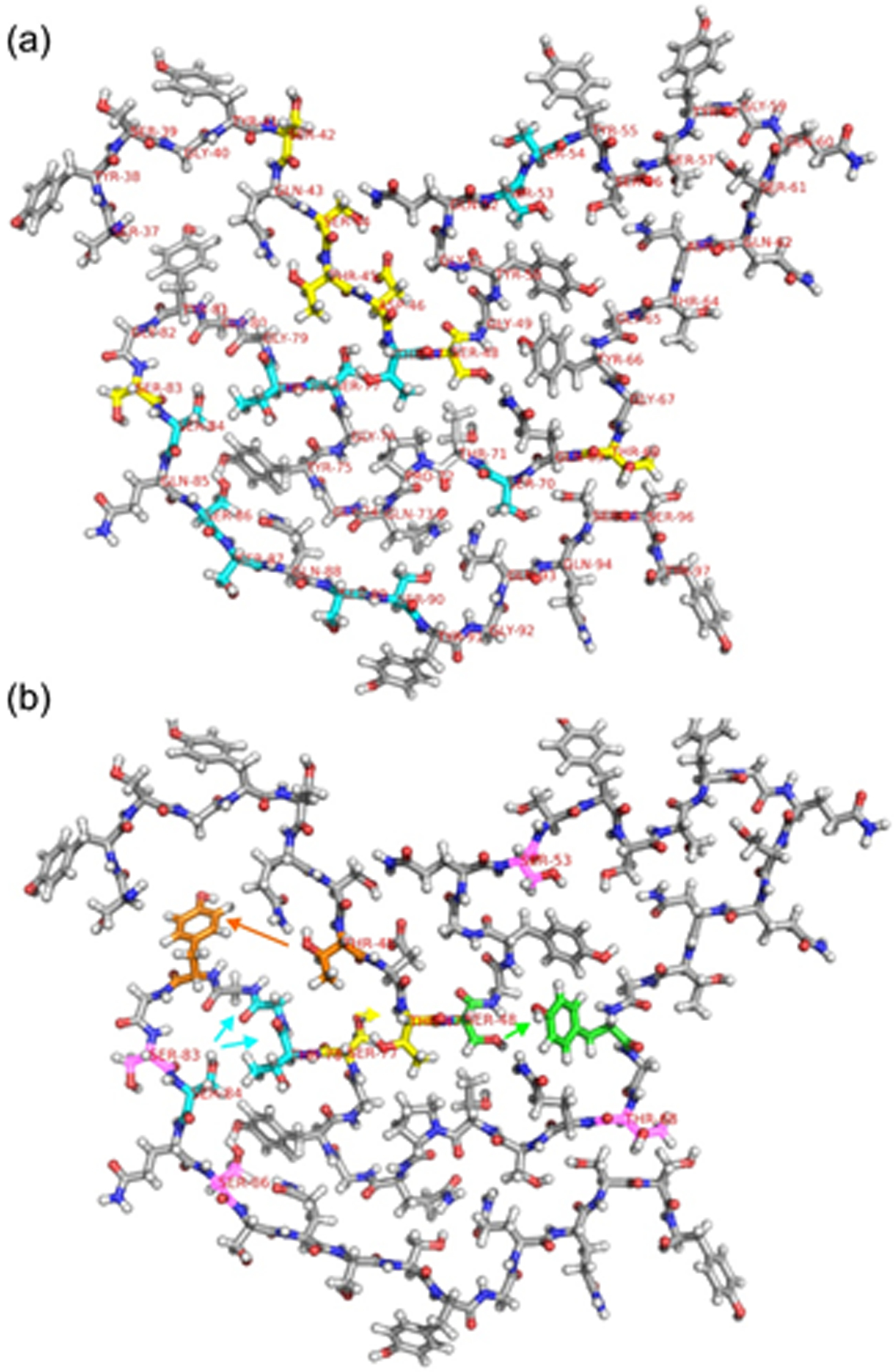
(a) One molecule from Protein Data Bank file 5W3N, with carbons of Ser, Thr, Asp residues that have correlations to the 1H chemical shift of water in the 3D HCC spectrum of 2H,15N,13C-FUS-LC fibrils (and also have 13C chemical shift assignments) shown in yellow. Carbons of Ser and Thr residues that have 13C chemical shift assignments, but do not have correlations to water, are shown in cyan. (b) One molecule from Protein Data Bank file 5W3N, shown with residue number labels on all Ser and Thr residues that have 1H chemical shift assignments for sidechain hydroxyl protons. Carbons of residues that are involved in inter-residue crosspeaks in 3D HCC or 3D H(H)CC spectra are colored orange, cyan, yellow, and green, with arrows indicating the direction of these crosspeaks from hydroxyl 1H chemical shifts to 13C chemical shifts. Based on the structural model, crosspeaks from hydroxyl 1H chemical shifts of T48 and S48 to aromatic 13C chemical shifts of Tyr residues are depicted as T48-Y81 and S48- Y66 crosspeaks. Carbons of Ser and Thr residues that have hydroxyl 1H chemical shift assignments but do not exhibit detectable inter-residue crosspeaks are colored pink.
