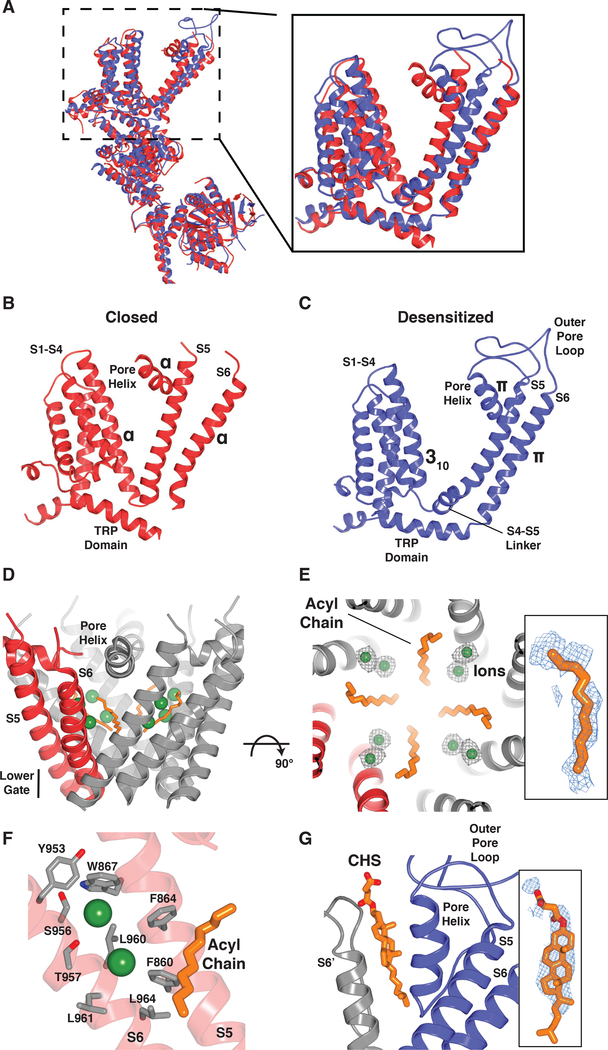
Fig. 4. Conformational changes associated with ligand binding to a shared pocket.
(A) Superimposition of the closed (TC-I 2014-bound; red) and desensitized (blue) TRPM8 structures. (B and C) Structural changes associated with TRPM8 channel gating. Transition from the closed (B) to desensitized (C) state is accompanied by formation of a S4-S5 linker, a local α-to-π-helical transition in S6, and rearrangement of the TRP domain, which alter the lower gate. (D) In the closed state, an ordered acyl chain (orange) is present along the ion permeation pathway, thereby increasing the hydrophobicity of the pore. Green spheres indicate ions observed in the vicinity of the acyl chains. (E) Close-up view, orthogonal to (D) with ion densities (gray mesh; 3σ contour). Inset shows density for acyl chain (blue mesh, 3σ contour). (F) Cation binding sites (green spheres) are in close proximity to negatively charged residues contributed by S6. Nitrogen, blue; oxygen, red. (G) In the desensitized state, a stabilizing lipid packs between the pore helix and S6 of the neighboring subunit (modeled as CHS). Inset shows lipid density (blue mesh, 4σ contour).