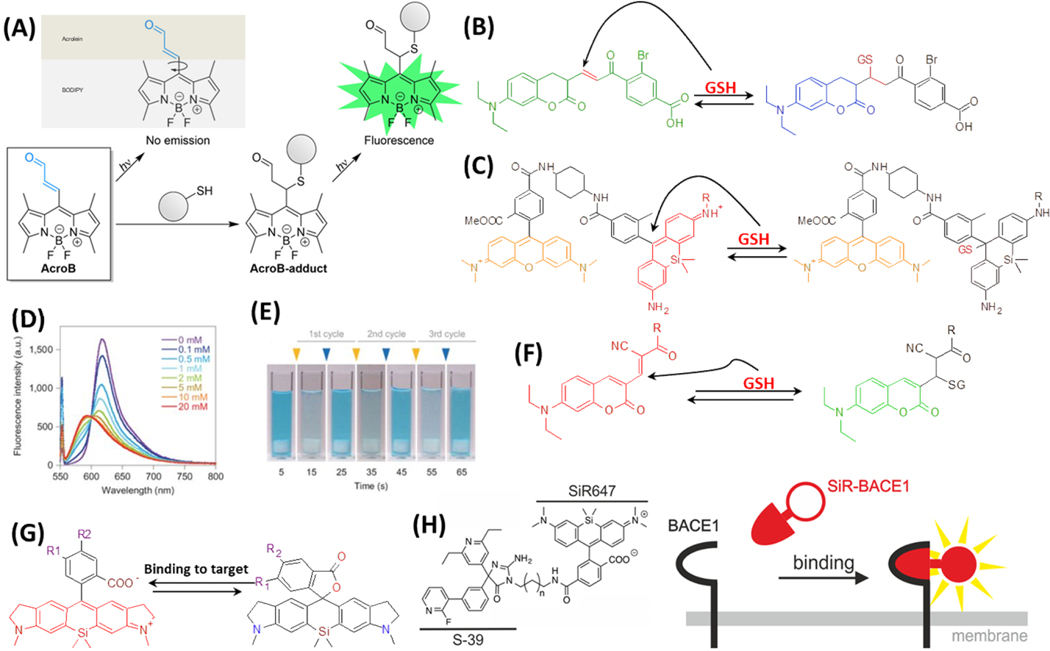
Figure 2.
(A) Molecule structure and mechanism of action of AcroB. Reproduced from Lincoln, R.; Greene, L. E.; Zhang, W.; Louisia, S.; Cosa, G. J. Am. Chem. Soc. 2017, 139, 16273–16281 (ref 51). Copyright 2017 American Chemical Society. Molecule structures and reactions of reversible fluorogenic probes for GSH: (B) TQ green, (C) SiR and TMR FRET system, and (F) QG-1. (D) The fluorescence intensity changes of SiM-TMR FRET system, with adding different concentration of GSH. (E) The visualization of reversible colour-changing of the SiM probe. GSH and NEM (N-ethylmaleimide) are added alternatively at 10-second intervals. The concentration of the dye is 20 μM. The final concentrations of GSH and NEM after each cycle are 5 mM. (D) and (E) are reprinted by permission from Macmillan Publishers Ltd: NATURE, Umezawa, K.; Yoshida, M.; Kamiya, M.; Yamasoba, T.; Urano, Y. Nat. Chem. 2017, 9, 279–286 (ref 55). Copyright 2017. (G) Examples of one SiR dye undergoing ground-state isomerization. R1 and R2 are possible sites to add targeting moieties for various applications. (H) Molecule structure and mechanism of action of SiR647 conjugated to S-39 that targets BACE1 protein. Reproduced from Karch, S.; Broichhagen, J.; Schneider, J.; Böning, D.; Hartmann, S.; Schmid, B.; Tripal, P.; Palmisano, R.; Alzheimer, C.; Johnsson, K.; Huth, T. J. Med. Chem. 2018, 61 (14), 6121–6139 (ref 62). Copyright 2018 American Chemical Society.