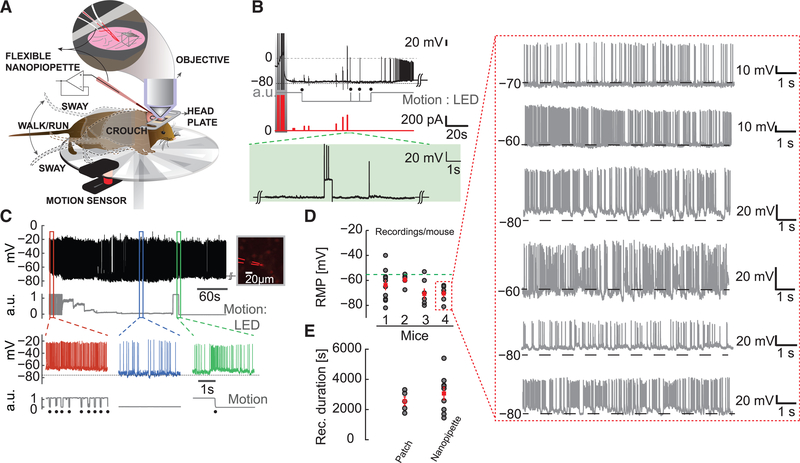
Figure 4. Targeted Intracellular Recordings from the Somatosensory Cortex in Awake Moving Mice.
(A) Schematic depicting blind and targeted intracellular recording using flexible nanopipettes in the awake head-fixed animal. The mouse is allowed to move on a circular wheel. The mouse exhibits various body movements: sway, crouch, walk, and run. The flexible nanopipette bends with this motion while maintaining intracellular access (inset).
(B) Typical blind recording performed ~800 μm deep in the visual cortex (V1). Intracellular break-in (type A) is elicited upon nanopipette stimulation (dark gray, shaded region) with a stable RMP of about −78 mV. Running and rest conditions (gray trace, black dots, digitized for clarity) are monitored by the motion sensor. Current injection (red trace) elicits clear AP activity (green dotted lines, shaded region) with a minimal increase in pipette resistance (residual unbridged resistance after cell entry). Note the stable intracellular baseline even in the presence of movement.
(C) Targeted intracellular recording (type A, black trace, top) from a layer 2/3 Pv interneuron (right, inset) in the motor cortex showing stable AP amplitude and steady RMP. Recordings last ~10 min to 1 hr in the presence of locomotion. Motion sensor (gray, raw data) indicates a rapid running movement (red region) followed by a gradual decrease, eventually coming to rest (blue region), and a subsequent movement again (green region, walk or run). Note the strong correlation between onset of movement and increase in AP firing rate (green trace).
(D) RMP for ten consecutive cells measured using a single nanopipette in each mouse. Entry in all cases was type A. Note the consistency in RMPs measured across multiple cells using the same electrode. The green dotted line indicates the −55 mV mark. This result clearly demonstrates that nanopipettes do not clog and can be repeatedly reused. The red dotted box shows the amplitude and stability of each recording from mouse 4 as an example.
(E) Duration of nanopipette recordings across 7 awake mice (n = 9 cells) compared to duration of recordings achieved using the whole-cell patch recordings in anaesthetized mice.
Data reflect mean ± SEM. Note the similarity in mean and maximal durations.