Abstract
The COVID-19 pandemic has spread across more than 200 countries and resulted in over 170,000 deaths. For unclear reasons, higher mortality rates from COVID-19 have been reported in men compared to women. While the SARS-CoV-2 receptor ACE2 and serine protease TMPRSS2 have been detected in lung and other tissues, it is not clear what sex differences may exist. We analyzed a publicly-available normal human prostate single-cell RNA sequencing dataset and found TMPRSS2 and ACE2 co-expressing cells in epithelial cells, with a higher proportion in club and hillock cells. Then we investigated datasets of lung epithelial cells and also found club cells co-expressing TMPRSS2 and ACE2. A comparison of ACE2 expression in lung tissue between males and females showed higher expression in males and a larger proportion of ACE2+ cells in male type II pneumocytes, with preliminary evidence that type II pneumocytes of all lung epithelial cell types showed the highest expression of ACE2. These results raise the possibility that sex differences in ACE2 expression and the presence of double-positive cells in the prostate may contribute to the observed disparities of COVID-19.
Keywords: COVID-19, SARS-CoV-2, TMPRSS2, ACE2, prostate, lung, sex
Introduction
As of April 23th, 2020, the COVID-19 pandemic has infected more than 2.5 million people across 213 countries and killed over 170,000 [1]. Despite there being approximately equal number of cases between men and women, emerging reports across countries indicate a higher mortality from SARS-CoV-2 in men than women, though the underlying reasons remain unclear [2]. The extent to which this disparity is due to biological rather than behavioral or comorbid sex differences is unknown.
The SARS-CoV-2 receptor, ACE2, and serine protease, TMPRSS2, are expressed in lung and other tissues implicated in the clinical manifestations of COVID-19. However, less is known about the exact cell types expressing ACE2 and TMPRSS2 that serve as cells of entry and pathogenesis for SARS-CoV-2 [3]. Intriguingly, TMPRSS2, which is one of the most dysregulated genes in prostate cancer, is highly expressed in human prostate epithelial cells and is androgen-responsive [4]. The presence of TMPRSS2 in the human prostate and its regulation by androgen raises the possibility that the prostate may be susceptible to SARS-CoV-2 infection, and that male-sex may be a biological risk factor.
Given the high expression of TMPRSS2 expression in the prostate, we investigated whether TMPRSS2 and ACE2 are co-expressed in human prostate epithelial cells, and compared our findings to expression levels found in five lung single-cell datasets. Lastly, we investigate sex differences in the expression of ACE2 and TMPRSS2 in lung epithelia.
Results
Using a publicly-available single-cell RNA sequencing dataset, we analyzed 24,519 epithelial cells from normal human prostates for expression of ACE2 and TMPRSS2 [5]. In this dataset, 0.32% of all epithelial cells (78 of 24,519), 0.47% of all stromal cells (10 of 2,113), 0.06% of endothelial cells (1 of 1,586) and 0.22% of leukocytes (1 of 459) expressed ACE2. 18.65% of all epithelial cells (4,573 of 24,519), 41.74% of all stromal cells (882 of 2,113), 16.71% of endothelial cells (265 of 1,586) and 52.07% of leukocytes (239 of 459) expressed TMPRSS2. Moreover, we found 30 cells that co-expressed ACE2 and TMPRSS2 (0.11% of epithelial cells, 0.10% of all cells) from numerous cell types in the prostate: 0.09% of stromal cells (2 of 2,113), 0.06% of endothelial cells (1 of 1,586), 0.03% of basal epithelial cells (5 of 18,439), 0.18% of luminal epithelial cells (4 of 2,238), 0.40% of hillock cells (10 of 2,530), and 0.61% of club cells (8 of 1,312) (Figure 1).
Figure 1.
Cell type distribution and top differentially expressed gene marker expression of the four datasets used in the current study. a. Normal human prostate epithelial cells [5] (BE: basal epithelial cells, LE: luminal epithelial cells). b. Human lung epithelial cells from the Vieira Braga et al. study [7]. c. Human lung epithelial cells from the Reyfman et al. study [8]. d. Human lung epithelial cells from the Habermann study [9]. e. Human lung epithelial cells from Raredon study [10]. Each dataset was re-clustered and annotated by different cell types, for which distribution was shown in the Uniform Manifold Approximation and Projection (UMAP). For each dataset, a dot plot was generated showing the percentage of expression and average expression level for the most differentially expressed genes in each cell type as well as ACE2 and TMPRSS2. The marker radius represents the percentage of expression and the color gradient represents the average expression level.
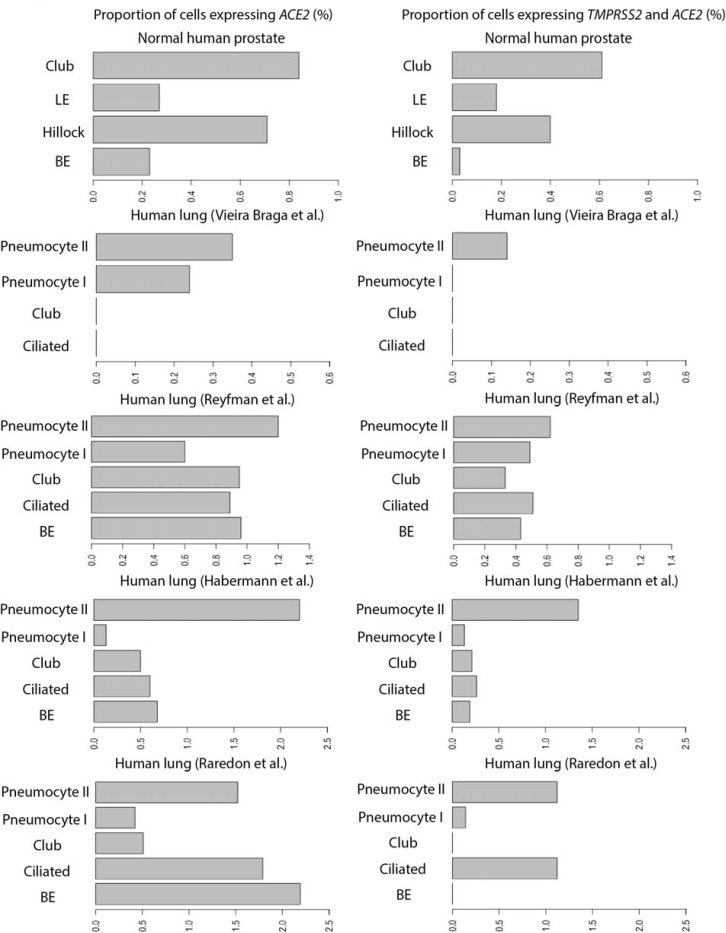
Prostate club cells were found to have the greatest proportion of double-positive cells in the human prostate, and these cells bear a strong resemblance to lung club cells [5]. To characterize ACE2 and TMPRSS2 expression in prostate club cells, we compared the expression levels of these genes in lung club cells from one mouse lung [6] and four human lung [7–10] single-cell datasets (Supplementary Table 1). We found a higher proportion of cells expressing TMPRSS2 than those expressing ACE2 (Figure 1) in all major lung epithelial cell types (basal epithelial cells, ciliated cells, club cells, type I and II pneumocytes). Specifically, in club cells, we detected the following proportions of double-positive cells in the datasets: 0.33% (7 of 2,113) of human lung club cells in the Reyfman et al. dataset [8]; 0.21% (3 of 1,410) of human lung club cells in the Habermann et al. dataset [9] (Figure 2); and 1.86% (48 of 2,578) of mouse lung club cells in the Montoro et al. dataset [6] (Supplementary Figure 1). Two other human lung datasets showed no such double-positive club cells [7,10] (Figure 2).
Figure 2.
Proportion of cells expressing ACE2, and co-expressing of TMPRSS2 and ACE2 (%) in each cell type (vertical axes) of the human prostate epithelial cells and four human lung epithelial cell datasets analyzed in the current study.
Lastly, to test sex differences in expression profiles of ACE2 and TMPRSS2, we compared ACE2 and TMPRSS2 expression differences within the integrated lung epithelial cells dataset stratified by sex [8,10] (Male: N = 12, Female: N = 19, Supplementary Table 1). Overall, there was no significant difference in TMPRSS2 expression between males and females in the human lung (Figure 3a). However, ACE2 expression was higher in males (log 2 normalized expression level: 0.019 in male vs 0.0068 in female, p < 0.001, Figure 3a).
Figure 3.
a. Comparison of TMPRSS2 and ACE2 expression level between females (N=19) and males (N=12) for all human lung epithelial cells (a) and by each epithelial cell type (b) within the integrated dataset (Supplementary Table 1). Statistical significance levels computed from an unpaired two-samples Wilcoxon test were indicated in the violin plots (NS: non-significant, *: p < 0.05, **: p < 0.01, ***: p < 0.001). c. Proportion of cells expressing ACE2, and co-expressing of TMPRSS2 and ACE2 (%) in each epithelial cell type and sex of the integrated dataset. Statistical significance levels computed from the Fisher’s exact test were indicated in the bar charts (NS: non-significant, ***: p < 0.001).
To characterize differences in cell types by sex, we then compared ACE2 and TMPRSS2 proportions of expression in the integrated dataset. We found higher proportions of type II pneumocytes in males that expressed ACE2 and co-expressed ACE2 and TMPRSS2 compared to type II pneumocytes in females (p < 0.001, Figure 3c). It is not clear if TMPRSS2 and ACE2 expression are regulated by the same process but our analysis reveals that their expression levels are positively correlated in lung cell lines (Supplementary Figure 2).
Discussion
In this study examining ACE2 and TMPRSS2 expression in prostate and lung epithelia, we highlight the prostate as a potential organ susceptible to SARS-CoV-2 infection. In human prostate cells, we found hillock and club cells as double-positive cells with the highest proportion of ACE2 and TMPRSS2 co-expression. Since both of these proteins are considered to be required for infection, these double-positive cells could potentially serve as reservoirs for SARS-CoV-2 infiltration and damage to the prostate; however, they constitute approximately 0.07% of all prostate epithelial cells. Interestingly, Henry and colleagues recently characterized the prostate club cell [5] as sharing a genetic signature similar to lung club cells [6]. Therefore, in order to better understand the prostate’s susceptibility to infection, we compared ACE2 and TMPRSS2 expression in both prostate and lung club cells. We found that 0.61% of prostate club cells co-express ACE2 and TMPRSS2, which correlate with the low numbers of lung club cells with ACE2 and TMPRSS2 co-expression across four human datasets. Compared to the proportion of ACE2 and TMPRSS2 co-expression in type II pneumocytes from prior reports (3.8%) [11], club cells have a markedly lower proportion of double-positive cells. Implications of SARS-CoV-2 infiltration of the prostate via club cells are unclear and warrant further investigation.
Here we also report differences in the co-expression of ACE2 and TMPRSS2 in type II pneumocytes by sex. In the integrated dataset with sex data reported, male type II pneumocytes possess a significantly greater proportion of cells expressing ACE2 and cells co-expressing ACE2 with TMPRSS2. We found no significant difference in TMPRSS2 expression between male and female lung epithelial cells. This finding is consistent with another recent report examining the same sex difference [12]. If these findings are reproducible with greater sample sizes, the greater proportion of ACE2 and TMPRSS2 co-expression in male type II pneumocytes may contribute to the associated disparities in COVID-19 pathogenesis. SARS-CoV-2-related alveolar damage has been implicated to primarily take effect via infiltration of type I and II pneumocytes and ciliated epithelial cells [13]. Type II pneumocytes perform essential functions for alveolar integrity via stem-cell properties and surfactant secretion [14], and their demise can contribute to alveolar collapse and respiratory failure. However, we caution against the generalizability of these findings due to the small sample size, lack of consensus among the data, and the lack of control for confounding variables that may modulate ACE2 or TMPRSS2 expression such as smoking [8,10].
Here we find that prostate epithelial cells co-express TMPRSS2 and ACE2 with the highest proportion found in hillock and club cells. Whether differences in TMPRSS2 and ACE2 expression mediate SARS-CoV-2 pathogenesis and whether androgen signaling can affect COVID-19 disease remain to be studied. Sex differences in TMPRSS2 and ACE2 expression could influence potential sites of disease and viral reservoirs. Sex differences in TMPRSS2 expression alone in the lung may not drive the higher burden of SARS-CoV-2 disease among men. However, our finding that distinct populations of cells in the prostate co-express TMPRSS2 and ACE2 raises the possibility that sex differences could influence COVID-19 disease pathogenesis in males differently than females. Further research into TMPRSS2 expression and its modulation within the lung and other relevant cell types that may impact ACE2 and SARS-CoV-2 pathogenesis is needed.
Methods
We adopted previously published single cell RNA-seq datasets of normal human prostate epithelial cells, mouse lung, and four human lung epithelial cells (Supplementary Table 1). Datasets were acquired using the raw count matrices made available by previous studies. For the human prostate, mouse, the Reyfman et al. and the Habermann et al. human lung datasets, cell types were annotated by respective cell type annotation metadata provided in the original studies and validated by top gene markers [5,6,8,9]. The cell type of the epithelial cell population in the Vieira Braga et al., dataset [7] was annotated by the top differentially expressed markers and validated by another previous study [15]. Similarly, the cell type of epithelial cell population in the Raredon et al. dataset [10] was annotated. Sample sex data were acquired for the Reyfman study and the Raredon study [8,10] but unavailable for the other human lung datasets [7,9]. All datasets were analyzed in R using the Seurat 3.1.4 package for visualization and comparison [16]. Gene expression in each dataset was normalized and scaled following the standard Seurat workflow, and sample batch effects were removed using the integration function implemented in Seurat 3.1.4 [16]. Cell distribution in each dataset was visualized in Uniform Manifold Approximation and Projection (UMAP), generated based on the principal component analysis and color-coded by different cell types annotation from the original study. The Reyfman et al. dataset and the Raredon et al. dataset were integrated following the Seurat 3.1.4 integration workflow to remove the batch effect from different samples and stratified by sex of samples reported in the original studies. Top differentially expressed gene markers were identified using the FindAllMarker function built within Seurat and ranked by the log2 fold change of expression [16]. The statistical significance of expression levels between males and females was computed from an unpaired two-samples Wilcoxon test and the proportions of expression between males and females were compared using Fisher’s Exact tests.
Supplementary Material
Acknowledgements
This work was funded in part by the Department of Defense W81XWH-17-PCRP-HD (F.W.H., H.S), the National Institutes of Health/National Cancer Institute P20 CA233255-01 (F.W.H., H.S), U19 CA214253 (F.W.H., H.S), and the Prostate Cancer Foundation (F.W.H.).
Footnotes
The authors have declared that no conflict of interest exists.
Reference
- [1].https://www.who.int/emergencies/diseases/novel-coronavirus-2019, accessed April 23, 2020
- [2].BMJ GH Blogs. Sex, gender and COVID-19: Disaggregated data and health disparities. BMJ Global Health blog; https://blogs.bmj.com/bmjgh/2020/03/24/sex-gender-and-covid-19-disaggregated-data-and-health-disparities/. Published March 24, 2020. [Google Scholar]
- [3].Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. March 2020. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Tomlins SA, Rhodes DR, Perner S, et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310(5748):644–648. doi: 10.1126/science.1117679 [DOI] [PubMed] [Google Scholar]
- [5].Henry GH, Malewska A, Joseph DB, et al. A Cellular Anatomy of the Normal Adult Human Prostate and Prostatic Urethra. Cell Rep. 2018;25(12):3530–3542.e5. doi: 10.1016/j.celrep.2018.11.086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Montoro DT, Haber AL, Biton M, et al. A revised airway epithelial hierarchy includes CFTR-expressing ionocytes. Nature. 2018;560(7718):319–324. doi: 10.1038/s41586018-0393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Vieira Braga FA, Kar G, Berg M, et al. A cellular census of human lungs identifies novel cell states in health and in asthma. Nat Med. 2019;25(7):1153–1163. doi: 10.1038/s41591-019-0468-5 [DOI] [PubMed] [Google Scholar]
- [8].Reyfman PA, Walter JM, Joshi N, et al. Single-Cell Transcriptomic Analysis of Human Lung Provides Insights into the Pathobiology of Pulmonary Fibrosis. Am J Respir Crit Care Med. 2019;199(12):1517–1536. doi: 10.1164/rccm.201712-2410OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Habermann AC, Gutierrez AJ, Bui LT, et al. Single-cell RNA-sequencing reveals profibrotic roles of distinct epithelial and mesenchymal lineages in pulmonary fibrosis. bioRxiv. September 2019:753806. doi: 10.1101/753806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Raredon MSB, Adams TS, Suhail Y, et al. Single-cell connectomic analysis of adult mammalian lungs. Sci Adv. 2019;5(12):eaaw3851. doi: 10.1126/sciadv.aaw3851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ziegler C, Allon SJ, Nyquist SK, et al. SARS-CoV-2 Receptor ACE2 Is an Interferon-Stimulated Gene in Human Airway Epithelial Cells and Is Enriched in Specific Cell Subsets Across Tissues. Rochester, NY: Social Science Research Network; 2020. doi: 10.2139/ssrn.3555145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Stopsack KH, Mucci LA, Antonarakis ES, Nelson PS, Kantoff PW. TMPRSS2 and COVID-19: Serendipity or opportunity for intervention? Cancer Discov. April 2020. doi: 10.1158/2159-8290.CD-20-0451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Rockx B, Kuiken T, Herfst S, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. April 2020. doi: 10.1126/science.abb7314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee DF, Salguero FJ, Grainger D, et al. Isolation and characterization of alveolar type II pneumocytes from adult bovine lung. Sci Rep. 2018. August 9;8(1):11927. doi: 10.1038/s41598-018-30234-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Travaglini KJ, Nabhan AN, Penland L, et al. A molecular cell atlas of the human lung from single cell RNA sequencing. bioRxiv. March 2020:742320. doi: 10.1101/742320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stuart T, Butler A, Hoffman P, et al. Comprehensive Integration of Single-Cell Data. Cell. 2019;177(7):1888–1902.e21. doi: 10.1016/j.cell.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.