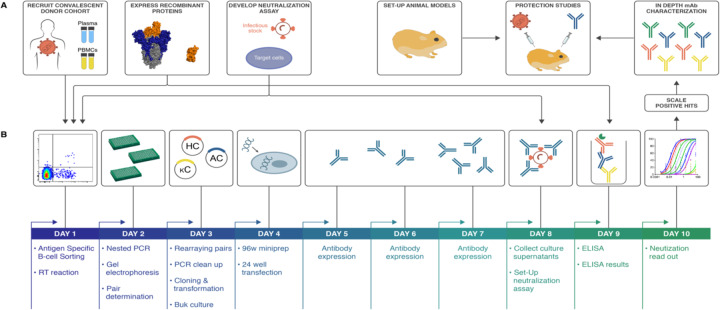
Figure 1. SARS-CoV-2 neutralizing antibody isolation strategy.
(A) A natural infection cohort was established to collect plasma and PBMCs samples from individuals who recovered from COVID-19. In parallel, functional assays were developed to rapidly screen all plasma samples for SARS-CoV-2neutralizing activity. SARS-CoV-2 recombinant surface proteins were also produced to use as baits in single memory B-cell sorting and downstream functional characterization of isolated mAbs. Finally, a hamster animal model was set-up to evaluate mAb passive transfer protection. (B) The standard mAb isolation pipeline was optimized to allow high-throughput amplification, cloning, expression and functional screening of hundreds of unpurified Ab heavy and light chain pairs isolated from each of several selected neutralizers in only 10 days. Selected pairs were scaled-up to purify IgG for validation and characterization experiments. The most potent neutralizing mAb was selected to evaluate protection in the Syrian hamster model.