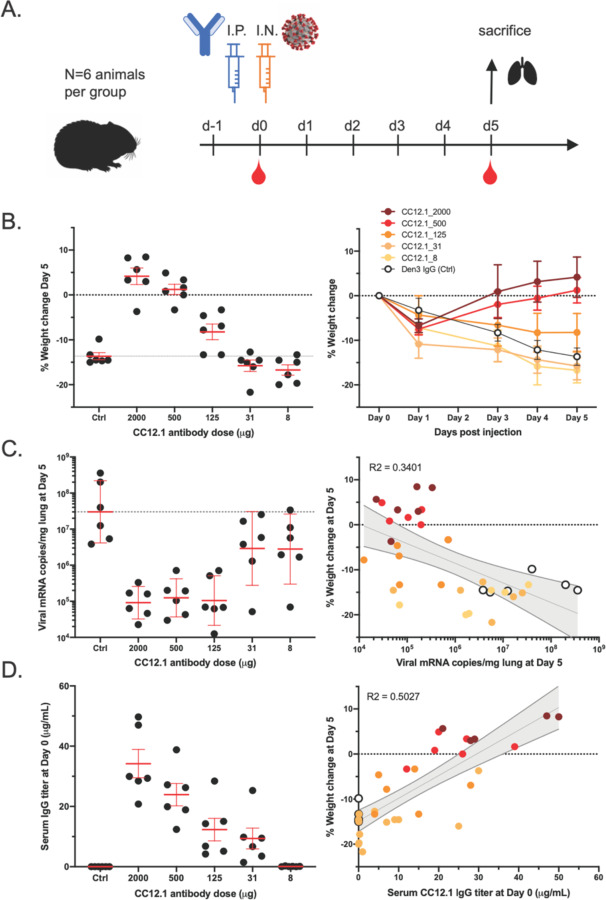
Figure 5. A potent SARS-CoV-2 RBD-specific neutralizing mAb protects against disease progression and lung viral burden in Syrian hamsters.
(A) SARS-CoV-2-specific human neutralizing mAb CC12.1 isolated from natural infection was injected intraperitoneally into Syrian hamsters at a starting dose of 2 mg/animal (on average 16.5 mg/kg) and subsequent serial 4-fold dilutions. Control animals received 2 mg of a dengue-specific human IgG1 (Den3). Each group of 6 animals were challenged intranasally 12h post-infusion with 1X106 PFU of SARS-CoV-2. Serum was collected at the time of challenge (Day 0) and Day 5, and their weight monitored as an indicator of disease progression. On day 5, lung tissue was collected for viral burden assessment. (B) Percentage weight change was calculated from day 0 for all animals at all time points. (C) Viral load as assessed by Q-PCR from lung tissue at day 5. (D) Serum titers of the passively administered mAb, as assessed by ELISA at the time of challenge (12h after i.p administration). Correlation analyses with 95% confidence intervals indicated in grey shade. R2 values are also indicated.