Figure 1.
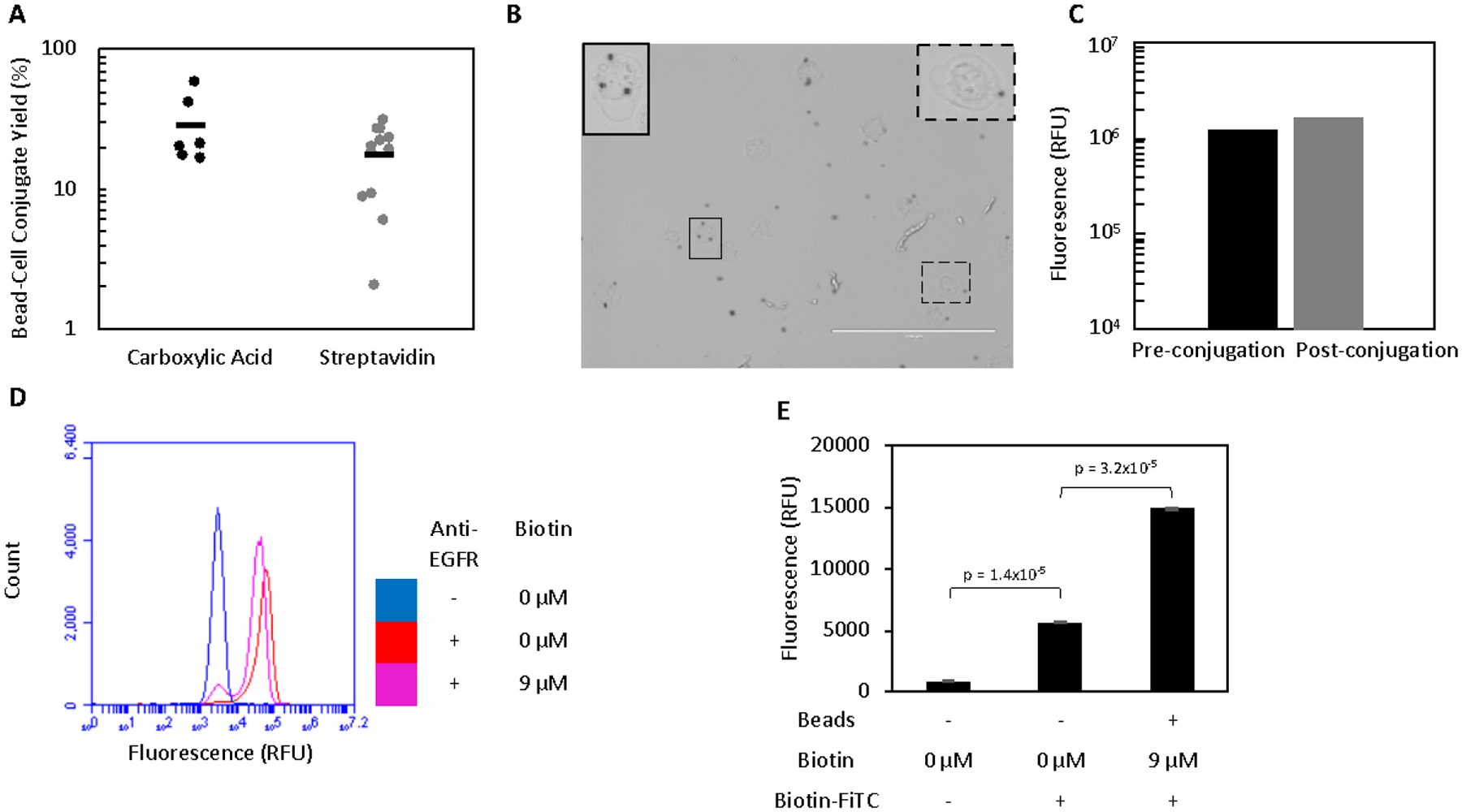
Mammalian cells can be efficiently conjugated to magnetic beads. A. Beads and A431 cells were incubated in a 1:1 ratio prior to washing on a magnet to remove all unbound cells. The yield of conjugates was quantified by flow cytometry. B. Bead-A431 conjugates (n = 39) were visualized to ensure proper conjugation. Carboxylic acid beads (black spheres) can be seen attached to the majority of A431 cells. Scale bar is 200 μm. Two cells (outlined) are enlarged and contrast-enhanced to show detail. C. A431 cells were labelled for with an anti-EGFR antibody and their fluorescence quantified by flow cytometry before and after conjugation to carboxylic acid beads to assess whether EGFR expression was extensively masked by conjugation to beads. D. A431 cells were labelled with an anti-EGFR antibody and their fluorescence quantified by flow cytometry before and after biotinylation to assess whether EGFR expression was extensively masked. A small number of cells had their expression masked by the labelling with 9 μM biotin. E. Unbiotinylated A431 cells with no beads and biotinylated A431 cells conjugated to streptavidin beads were labelled with biotin-FITC and the fluorescence of the cell population (as gated by scatter) was analyzed to detect the presence of streptavidin beads attached to A431 cells. While biotin-FITC significantly labels the cell surface in the absence of streptavidin beads, a further significant increase in signal is observed after conjugation occurs. Fluorescence is presented as mean ± standard error of three trials.
