Figure 9.
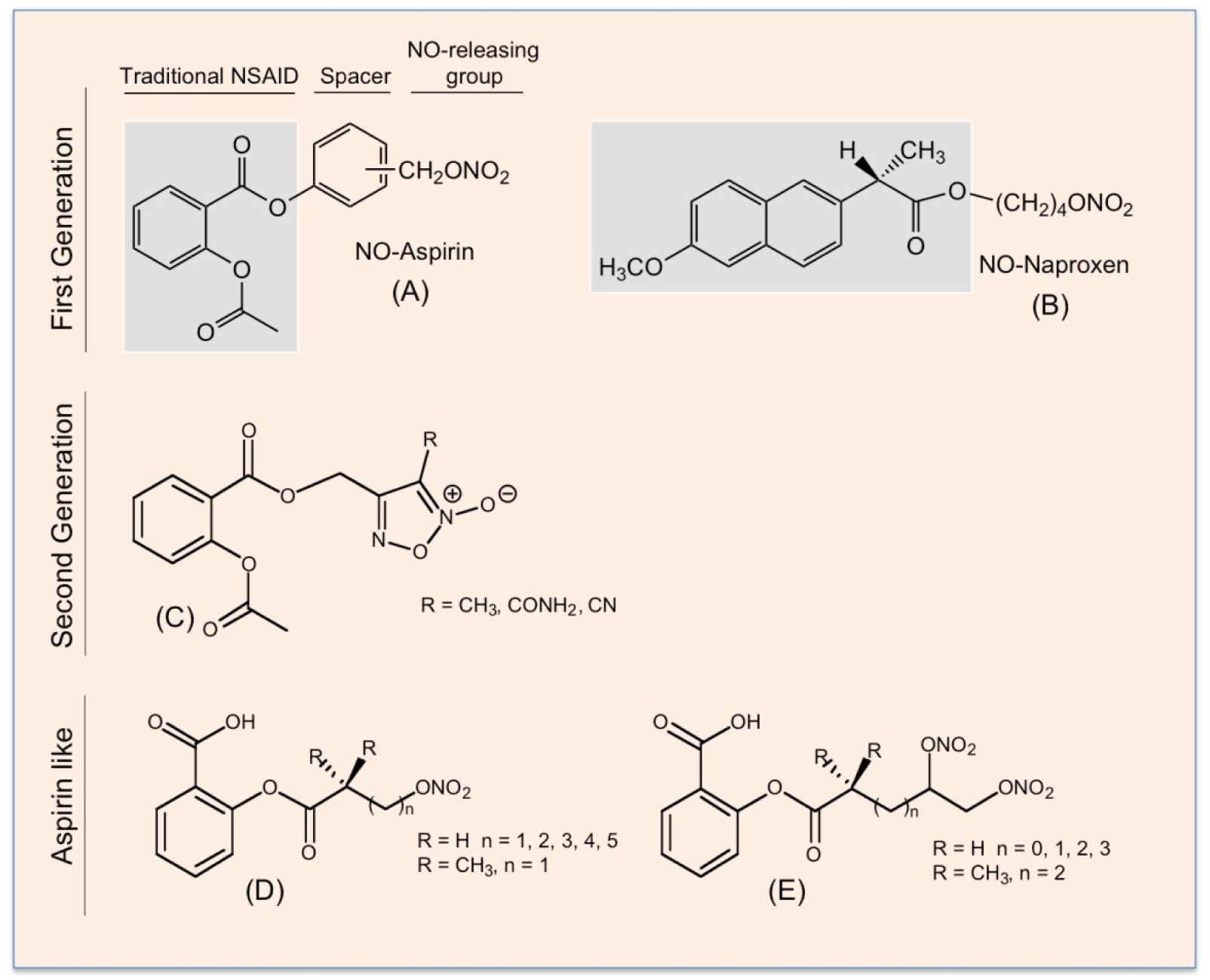
The chemical structures of first- and second-generation NO-NSAIDs and NO-donor “aspirin-like” compounds. The traditional NSAIDs, aspirin (A) and naproxen (B), are shown in the shaded boxes; the spacer molecule links the traditional NSAID to –ONO2, which can release NO. A second generation of NO-releasing aspirin in which a furoxan derivative is the NO donor (C). In the “aspirin-like” compounds, the acetyl group on the aspirin has been replaced by acyl groups containing nitroxy NO-releasing moieties, (D) and (E).
