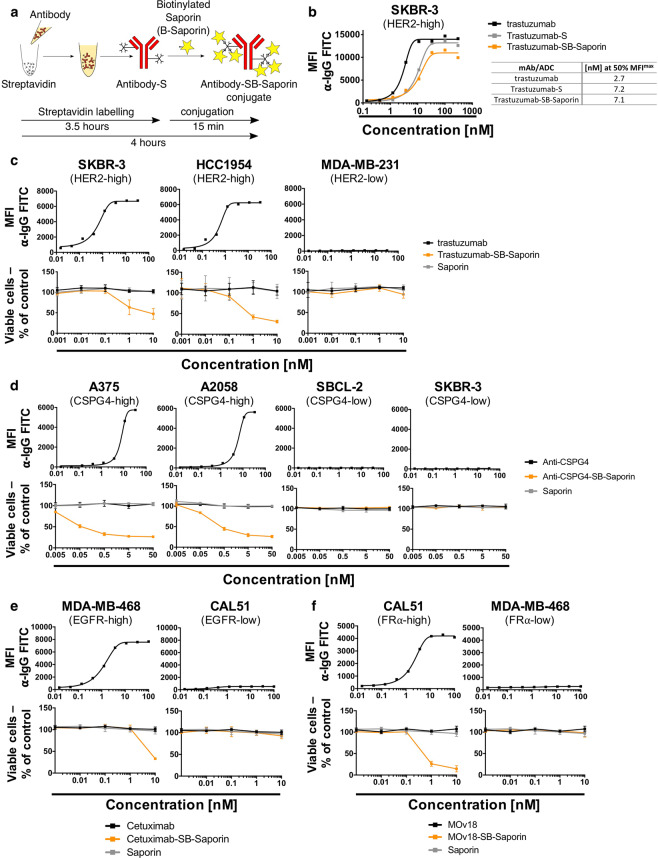
Figure 1.
Antibody Streptavidin linked to Biotin-Saporin as payload can be used to investigate antibody internalization (a) Schematic of Antibody-SB-Saporin conjugate generation. Streptavidin is conjugated randomly to lysine residues of the antibody with an average of two molecules of streptavidin per antibody. Streptavidin-linked antibody was conjugated to Monobiotin-Saporin at a molar ratio of 1:6. (b) Binding assay of trastuzumab, Trastuzumab-SB-Saporin and Trastuzumab-S to Her2-high SKBR-3 breast cancer cells and their concentrations at 50% of the maximum mean fluorescence intensity. (c–f) Top panels, target antigen recognition by naked antibodies, anti-Her2 trastuzumab (c), anti-CSPG4 (d), anti-EGFR Cetuximab (e), anti-FRα MOv18 (f) on cancer cell lines expressing different target antigens expression (Her2 (c), CSPG4 (d), EGFR (e), FRα (f)). Bottom panels (c–f), investigation of cell viability upon treatment with the naked antibody (black), antibody-Streptavidin-Biotin-Saporin conjugate (orange) or Saporin alone (grey). The ribosome inhibitor Saporin, unable to enter the cell alone, can be used to investigate antibody internalization by measuring viability (MTS) of antibody-SB-Saporin conjugate-treated cells. N = 1 for all binding assays and N = 3-4 independent experiments for MTS studies. All experiments were performed in triplicate, error bars represent Standard Deviation (SD).