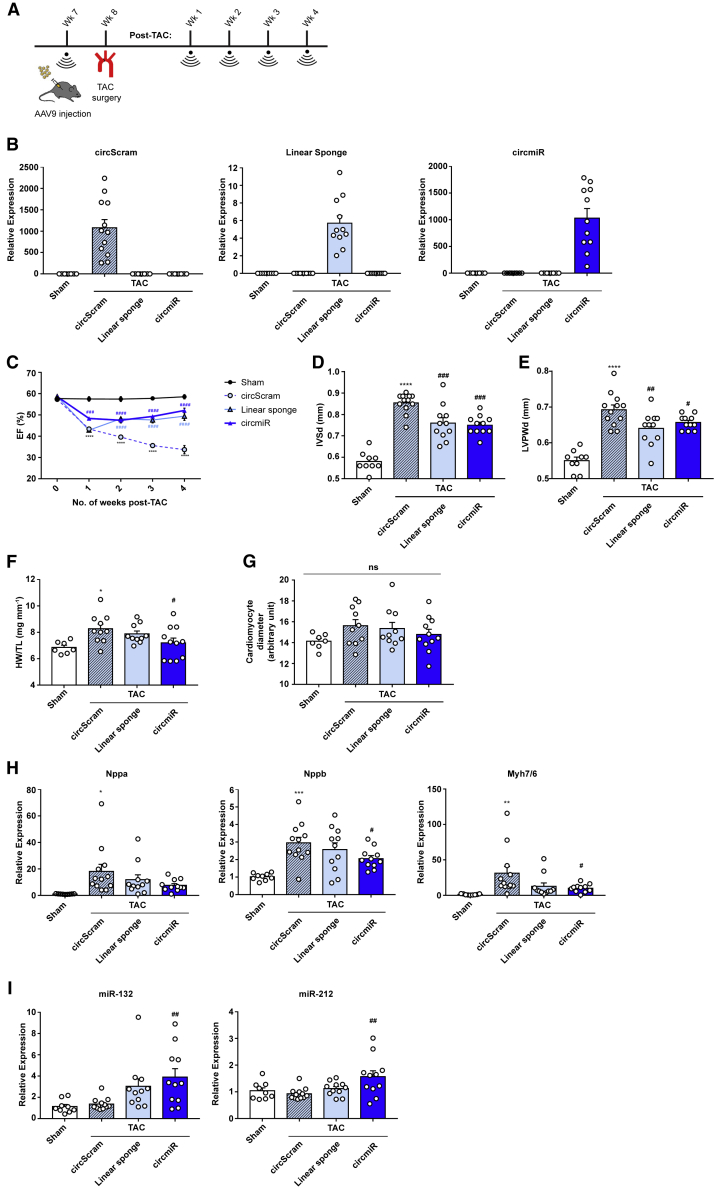
Figure 3.
circmiR Therapy Attenuates Pressure Overload-Induced Hypertrophy
(A) Experimental strategy to test circmiR therapeutic or circScram/linsp control constructs in vivo by AAV9 injection to 7-week-old mice. TAC surgery was performed one week later. Weekly echocardiography was conducted up to 4 weeks post TAC surgery before sacrifice. (B) Expression abundance of products from AAV construct circScram, linsp, and circmiR in isolated cardiomyocytes using qPCR. (C–E) Echocardiographic analysis of (C) ejection fraction, (D) interventricular septal thickness, and (E) left ventricular posterior wall thickness in circScram, linsp, and circmiR injected mice and sham-operated controls. (F) Heart weight to tibia length ratios and (G) cardiomyocyte diameter measured by immunofluorescence analysis of WGA and cTnI staining to visualize cell membrane and cardiomyocytes respectively in circScram, linsp, and circmiR injected mice and sham-operated controls. (H) Expression levels of cardiac stress response genes Nppa, Nppb, and Myh7/Myh6 ratio in isolated cardiomyocytes using qPCR. (I) miR-132 and miR-212 expression levels in isolated cardiomyocytes using qPCR. For all data, circScram (n = 12), circmiR (n = 11), linsp (n = 11), and sham (n = 9) except (F) and (G) where circScram (n = 10), circmiR (n = 11), linsp (n = 10) and sham (n = 7). ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 (sham versus circScram). #p < 0.05, ##p < 0.01, and ###p < 0.001 (circScram versus circmiR/linsp). One-way ANOVA with Benjamini-Hochberg adjustment.