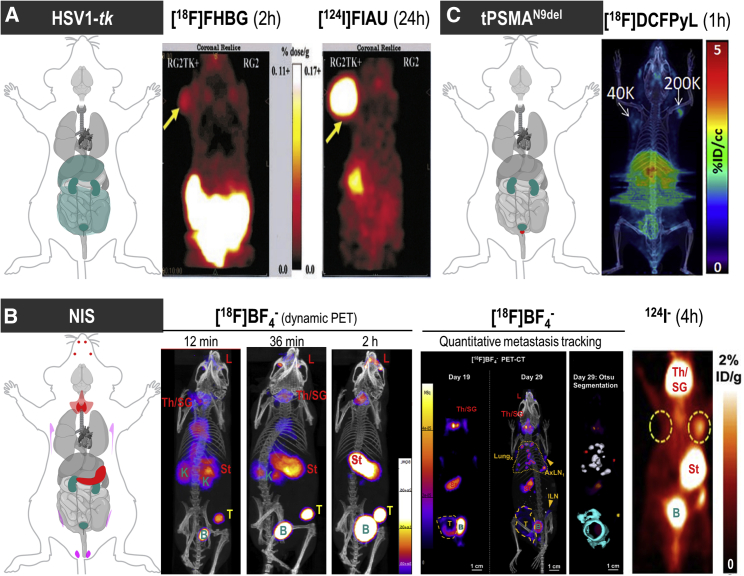
Figure 3.
Background Considerations for Foreign and Host Radionuclide Reporters
(A) HSV1-tk as an example of a foreign reporter is not expressed endogenously in healthy mammals. However, this does not mean that the radiotracer to detect HSV1-tk-expressing cells is excluded from background uptake in other mammalian cells/organs or from generating signals during excretion (dark cyan in cartoon). Moreover, it is fundamental for radionuclide imaging that a contrast between background signal and signal arising from reporter-expressing cells (by one of the molecular imaging mechanisms [Figure 1C]) is generated through tissue clearance of radiotracer molecules. Radiotracers can thus affect background differently across different organs as shown here for two different PET radiotracers for HSV1-tk. Images are reproduced from a study comparing HSV1-tk radiotracer performance,143 with yellow arrows pointing toward the regions of interest in this study (tumors). Here, the other anatomical sites showing signals are of note (hepatobiliary and renal excretion for [18F]FHBG and uptake into the stomach for [124I]FIAU). (B) NIS is an example of a host reporter and consequently is expressed endogenously in some organs; NIS is highly expressed in the thyroid and stomach (red), precluding cell tracking from these organs, and at low levels in testes (♂, pink), mammary (♀, pink), and salivary and lacrimal glands (light red). Images shown are from three different studies using varying PET radiotracers for NIS. (B) Left: image demonstrates how [18F]BF4−in vivo distribution changes over time (female mouse with mammary tumor indicated by a yellow “T”; for details, Diocou et al.127). (B) Middle: images shown demonstrate metastasis tracking over time and exquisite resolution and sensitivity of NIS-PET imaging for metastasis tracking. They also demonstrate the necrotic tumor core, which is not imaged by NIS due to its favorable dependence on cellular energy for function, thereby reflecting cell viability. An example of Otsu image segmentation is shown to the right, which is the basis for quantitation (for details, see Volpe et al.33). Further annotations are endogenous signals from thyroid and salivary glands (Th/SG), stomach (St), and lacrimal glands (L). (B) Right: this image is reproduced from a study elucidating the detection sensitivity of reporter-expressing engineered primary T cells128 with annotations the same as in the middle images. In both cases radiotracer excretion also leads to signals, in the case of these NIS tracers only from the renal excretion system (K, kidneys, B, bladder). (C) CAR-Ts were engineered to express the tPSMAN9del reporter and administered to NSG mice at the indicated numbers (in 50 μL of 50% Matrigel; white arrows). Imaging with the radiotracer [18F]DCFPyL resulted in CAR-T detection. Notably, images are not free of background despite PSMA endogenous expression limited to the prostate (red area in cartoon). This is because radiotracer clearance was incomplete at the point of imaging. To improve the display contrast of the in vivo images, the authors masked relatively high renal radiotracer uptake using a thresholding method. For experimental details, see Minn et al.29 [All data images in this figure are reproduced with minor modifications from the publications mentioned in the legend, with permission from corresponding publishers.]