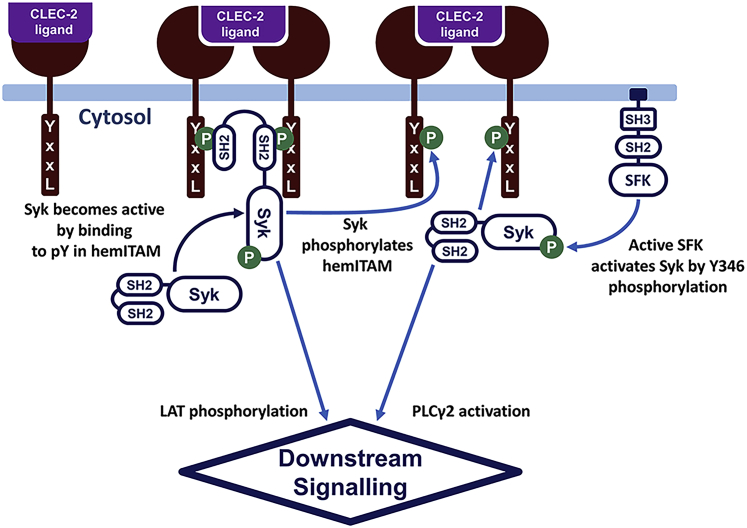
Figure 1.
CLEC-2-induced signaling in blood platelets. In resting platelets, relatively few Syk kinases are active because of the low SFK activity. Upon ligation of the platelet CLEC-2 and CLEC-2 cluster formation, tyrosine residues in the CLEC-2 cytoplasmic domain (YxxL sequence, hemITAM) become phosphorylated by Syk kinases. Nonactive Syk kinases bind to phosphorylated hemITAM with its SH2 domains and become active via trans-autophosphorylation. Accumulation of the active Syk results in downstream platelet activation and calcium signaling. To see this figure in color, go online.