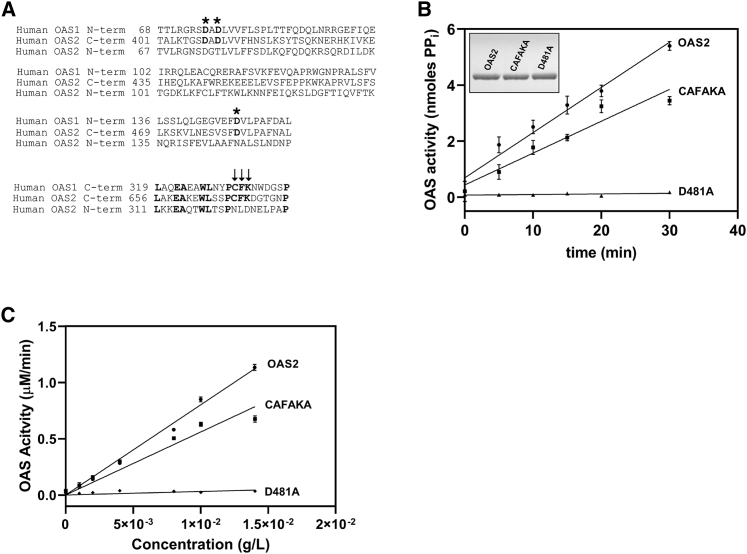
Figure 5.
Characterization of OAS2 mutants. (A) MUSCLE sequence alignment is shown. The sequence homology of OAS enzymes near the carboxyl terminal shows the conserved CFK residues represented by arrows, and the conserved aspartic acid residues are indicated by asterisks. (B) OAS2 activation comparison is shown. Activation assay (refer to Materials and Methods) comparing the activity (nmoles of PPi) of OAS2 and mutants D481A and CAFAKA is shown. SDS-PAGE analysis of purified OAS2, D481A, and CAFAKA is shown (inset). (C) OAS2 activation assay at varying concentrations (0.0–0.0143 g/L) is shown, comparing the activity (μM/min) of OAS2 and D481A and CAFAKA mutants. Error bars represent standard deviation from three replicates.