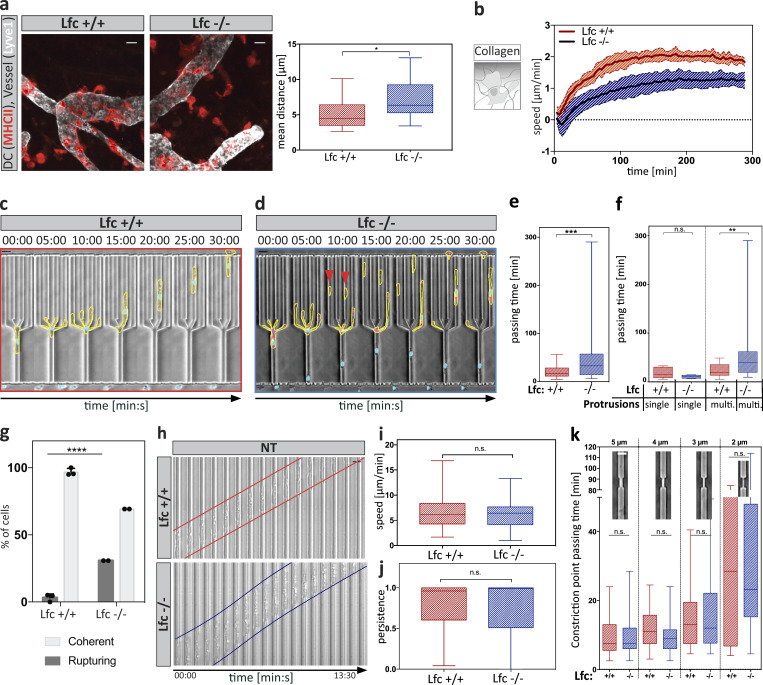
Figure 6.
Loss of Lfc causes DC entanglement. (a) In situ migration of exogenous DCs on a mouse ear sheet. Lymphatic vessels were stained for Lyve-1 and DCs with TAMRA. Right panel iindicates the mean distance of cells from lymphatic vessels. Per experiment, two mouse ears with two fields of view were analyzed from n = 4 experiments. Boxes extend from 25th to 75th percentiles. Whiskers span minimum to maximum values. Annotation above columns indicates results of unpaired Student’s t test; *, P ≤ 0.05. Scale bar, 100 µm. (b) Automated analysis of y-directed migration speed within a collagen network along a soluble CCL19 gradient. Plot shows mean population migration velocities over time ± SD from N = 7 experiments. (c) Time-lapse sequence of a wild type littermate control cell migrating within a path choice device. Scale bar, 10 µm. (d) Time-lapse sequence of an Lfc−/− cell migrating within a path choice device. Red arrowheads denote multiple rupturing events of an individual cell. Scale bar, 10 µm. (e) Junction point passing times of Lfc+/+ (n = 79 cells from N = 3 experiments) and Lfc−/− (n = 49 cells from N = 2 experiments) DCs. Boxes extend from 25th to 75th percentiles. Whiskers span minimum to maximum values. Annotation above columns indicates results of unpaired Mann-Whitney test; ***, P ≤ 0.001. See also Video 9. (f) Junction point passing times depending on presence of single non-competing or multiple (multi.) competing protrusions per cell of Lfc+/+ (n = 37 cells from N = 3 experiments) and Lfc−/− (n = 46 cells from N = 2 experiments) DCs. Boxes extend from 25th to 75th percentiles. Whiskers span minimum to maximum values. Annotation above columns indicates results of Kruskal-Wallis with Dunn’s test; **, P ≤ 0.01. (g) Frequency of cell rupturing events during migration within path choice device of Lfc+/+ (n = 79 cells ± SD from N = 3 experiments) and Lfc−/− (n = 52 cells ± SD from N = 2 experiments) DCs. Annotation above columns indicates results of two-way ANOVA with Sidak’s test; ****, P ≤ 0.0001. (h) Migration of DCs within straight microchannels. Cell edges are indicated in red (Lfc+/+) and blue (Lfc-/-). NT, non-treated. Scale bar, 10μm. (i) Migration speed of Lfc+/+ and Lfc−/− DCs within straight microchannels of n = minimum of 80 cells per condition from N = 5 experiments. Boxes extend from 25th to 75th percentiles. Whiskers span minimum to maximum values. Annotation above columns indicates results of one-way ANOVA. (j) Migratory persistence of Lfc+/+ and Lfc−/− DCs within straight microchannels of n = minimum of 80 cells per condition from N = 5 experiments. Boxes extend from 25th to 75th percentiles. Whiskers span minimum to maximum values. Annotation above columns indicates results of Kruskal-Wallis with Dunn’s test. (k) Single constriction passing times of Lfc+/+ (n = 114 cells from N = 3 experiments) and Lfc−/− (n = 195 cells from N = 3 experiments) DCs. Boxes extend from 25th to 75th percentiles. Whiskers span minimum to maximum values. Annotation above columns indicates results of Kruskal-Wallis with Dunn’s test. n.s., not significant.