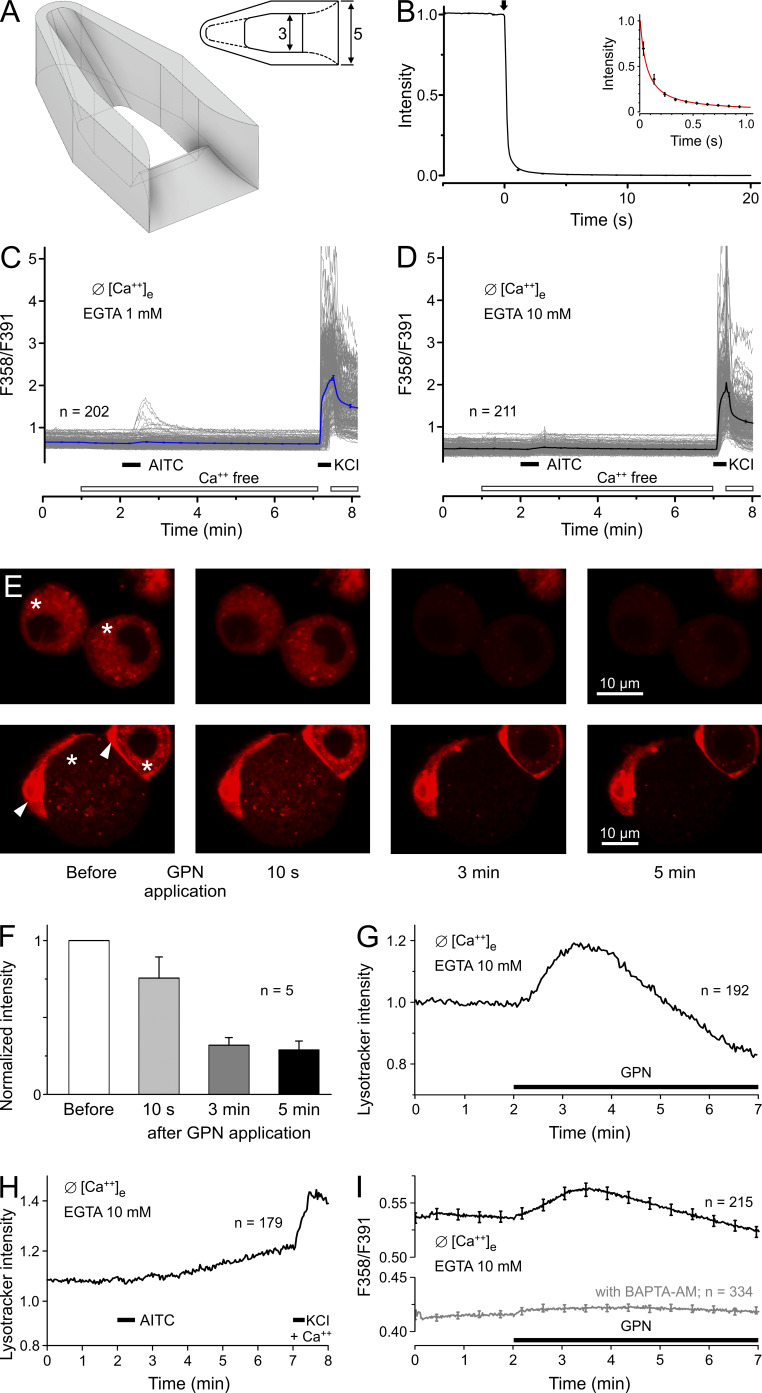
Figure 3.
After 1 min without external calcium, DRGs respond minimally in 1 mM EGTA but not in 10 mM EGTA. (A) Schematic drawing of the purpose-designed perfusion chamber generated by stereolithography using the 3D printer Form 2 (Formlabs Boston/Berlin) with 25-µm resolution and Fusion 360 software. The 2D view from the top provides dimensions in millimeters; the perfusion capillary with 1.5-mm outer diameter was placed at the left end. (B) The intensity time course of transmitted light after a switch (arrow) from a clear solution to one containing methylene blue was recorded seven times at 10 Hz. The solution was gravity driven at a flowrate of 1 ml/min and removed by a suction tube placed next to the outlet. The inset shows a power-law fit to the curve during the first second. (C) DRGs were measured 4–6 h after plating using the described perfusion chamber. The solution in the chamber was changed from calcium-containing to calcium-free 1 min before exposure to 100 µM AITC. The nominally calcium-free solution contained EGTA 1 mM. (D) 10 mM EGTA eliminated the responses to AITC. (E) DRG cells, neurons of different size (labeled *). and attached satellite cells (labeled with arrowheads), were loaded with LysoTracker and imaged by confocal microscopy under nominally calcium-free conditions (+ 10 mM EGTA). Images were acquired before and during 200 µM GPN application as indicated. The images show the rapid fading of pH-dependent fluorescence in four representative neurons, indicating lysosome disruption. (F) The normalized LysoTracker fluorescence intensity of five neurons dropped upon GPN application to ~25% within 5 min. (G) The same protocol was applied using wide-field microfluorimetry, allowing observation of more neurons. After a transient increase under GPN, LysoTracker fluorescence faded, indicating mass disruption of lysosomes. (H) Neurons stained with LysoTracker show no visible effect of 30-s AITC application (100 µM) in the absence of extracellular calcium. (I) Fura-2 calcium imaging reveals the small intracellular release of calcium ions from disintegrating lysosomes evoked by 200 µM GPN. Neurons pretreated with the intracellular calcium chelator BAPTA-AM for 30 min showed no response to GPN application. Error bars represent SEM values of the counted (n) neurons.