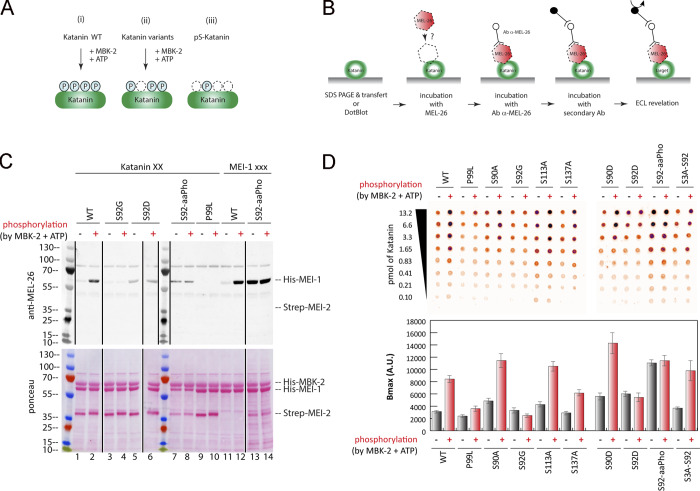
Figure 4.
Site-specific MEI-1 phosphorylation at S92 increases MEI-1 affinity for MEL-26. (A) Schematic of the different phosphorylated forms (P, phosphate) of Katanin (green oval) used in the assay. (Ai) WT Katanin phosphorylated by MBK-2. (Aii) Katanin with site-specific substitutions replacing serine by nonphosphorylatable alanine. (Aiii) Site-specific Katanin phosphorylation by genetic code expansion in E. coli. (B) Schematics of the different steps of the Far-Western blot method used to detect the interaction between MEI-1 and MEL-26. Purified Katanin (green oval) was separated on SDS-PAGE and transferred to PVDF membrane (denatured as in C) or directly spotted on nitrocellulose membrane (native as in D). The membrane was then incubated with purified MEL-26 (red hexagon). After extensive washing of the membrane, the interaction between MEL-26 and MEI-1 was detected using MEL-26 antibodies and classic ECL revelation. (C) MEL-26–Katanin interaction detected by Far-Western ligand binding assay after separation of Katanin or MEI-1 using SDS-PAGE (denaturing conditions). Top panel corresponds to MEL-26 detection via specific antibody against MEL-26 and ECL revelation. Bottom panel shows the same membrane stained with Ponceau Red to show protein loading. Experiment was performed at least three times, and the membranes presented are representative of the results. (D) MEL-26–Katanin interaction detected by Far-Western ligand binding assay after spotting decreasing amounts (top > down) of Katanin on nitrocellulose membrane (native conditions). Histogram summary of the maximal binding (Bmax) values extracted from the fitted binding curve are presented in Fig. S2.